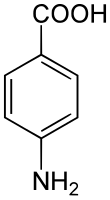
4-Aminobenzoic acid
[7] Many bacteria, including those found in the human intestinal tract such as E. coli, generate PABA from chorismate by the combined action of the enzymes 4-amino-4-deoxychorismate synthase and 4-amino-4-deoxychorismate lyase.
[9] The potassium salt is used as a drug against fibrotic skin disorders, such as Peyronie's disease, under the brand name Potaba.
[10] PABA is also occasionally used in pill form by sufferers of irritable bowel syndrome to treat its associated gastrointestinal symptoms, and in nutritional epidemiological studies to assess the completeness of 24-hour urine collection for the determination of urinary sodium, potassium, or nitrogen levels.
This makes it easier for the electrons in PABA to transition to a higher energy state upon absorbing light.
[18] However, animal and in vitro studies in the early 1980s suggested PABA might increase the risk of cellular UV damage.
[20] However, water-insoluble PABA derivatives such as padimate O are currently used in some cosmetic products including mascara, concealer, and matte lipsticks.
[21] As of 2008, the advancement of new sunscreen is focused on developing a broad spectrum of active ingredients that provide consistent protection across all wavelengths, including UVA.
Researchers are considering the PABA–TiO2 Hybrid Nanostructures that result from the method of aqueous in situ synthesis with PABA and TiO2.