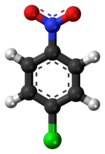
4-Nitrochlorobenzene
4-Nitrochlorobenzene is prepared industrially by nitration of chlorobenzene: This reaction affords both the 2- and the 4-nitro derivatives, in about a 1:2 ratio.
The electron-withdrawing nature of the appended nitro-group makes the benzene ring especially susceptible to nucleophilic aromatic substitution, unlike related chlorobenzene.
Thus, the strong nucleophiles hydroxide, methoxide, fluoride, and amide displace chloride to give respectively 4-nitrophenol, 4-nitroanisole, 4-fluoronitrobenzene, and 4-nitroaniline.
Reductive alkylation of the nitro group affords secondary aryl amines, which are useful antioxidants for rubber.
[7] The Occupational Safety and Health Administration set a permissible exposure limit of 1 mg/m3 The American Conference of Governmental Industrial Hygienists recommends an airborne exposure limit of 0.64 mg/m3 over a time-weighted average of eight hours.