Nucleophilic aromatic substitution
In order to attack the C atom, the nucleophile must approach in line with the C-LG (leaving group) bond from the back, where the benzene ring lies.
The resulting intermediate, named the Meisenheimer complex (2a), the ipso carbon is temporarily bonded to the hydroxyl group.
Since 2,4-dinitrophenol is in a lower energy state, it will not return to form the reactant, so after some time has passed, the reaction reaches chemical equilibrium that favors the 2,4-dinitrophenol, which is then deprotonated by the basic solution (4).
The formation of the resonance-stabilized Meisenheimer complex is slow because the loss of aromaticity due to nucleophilic attack results in a higher-energy state.
Recent work indicates that, sometimes, the Meisenheimer complex is not always a true intermediate but may be the transition state of a 'frontside SN2' process, particularly if stabilization by electron-withdrawing groups is not very strong.
As such, the following pattern is seen with regard to halogen leaving group ability for SNAr: F > Cl ≈ Br > I (i.e. an inverted order to that expected for an SN2 reaction).
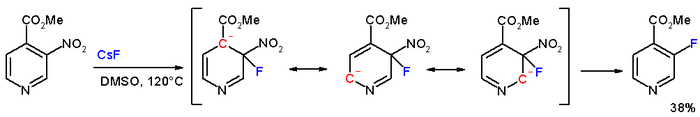
Nucleophilic aromatic substitution is not limited to arenes, however; the reaction takes place even more readily with heteroarenes.
[6] In the compound methyl 3-nitropyridine-4-carboxylate, the meta nitro group is actually displaced by fluorine with cesium fluoride in DMSO at 120 °C.
[8] With carbon nucleophiles such as 1,3-dicarbonyl compounds the reaction has been demonstrated as a method for the asymmetric synthesis of chiral molecules.