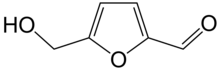
Hydroxymethylfurfural
Methoxymethylfurfural Hydroxymethylfurfural (HMF), also known as 5-(hydroxymethyl)furfural, is an organic compound formed by the dehydration of reducing sugars.
[4][5] It is a white low-melting solid (although commercial samples are often yellow) which is highly soluble in both water and organic solvents.
The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups.
[8][9] It is also produced industrially on a modest scale[10] as a carbon-neutral feedstock for the production of fuels[11] and other chemicals.
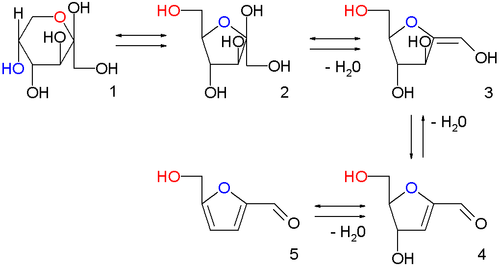
[13] This remains the classical route, with 6-carbon sugars (hexoses) such as fructose undergoing acid catalyzed poly-dehydration.
Similar chemistry is seen with 5-carbon sugars (pentoses), which react with aqueous acid to form furfural.
[10] Numerous synthetic technologies have been developed, including the use of ionic liquids,[17][18] continuous liquid-liquid extraction, reactive distillation and solid acid catalysts to either remove the HMF before it reacts further or to otherwise promote its formation and inhibit its decomposition.
[20][21] HMF can be converted to 2,5-dimethylfuran (DMF), a liquid that is a potential biofuel with a greater energy content than bioethanol.
Here, as well as in vinegars, jams, alcoholic products or biscuits, HMF can be used as an indicator for excess heat-treatment.
Adding bases such as soda ash or potash to neutralize the HFCS slows the formation of HMF.
The daily intake of HMF may underlie high variations due to individual consumption-patterns.
Photometric test may be unspecific as they may detect also related substances, leading to higher results than HPLC-measurements.