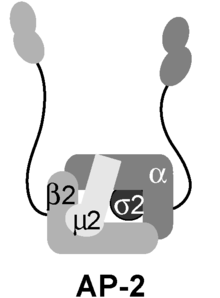
AP2 adaptor complex
The AP2 adaptor complex is a multimeric protein that works on the cell membrane to internalize cargo in clathrin-mediated endocytosis.
In its inactive state, the complex experiences a conformational change that causes both sites to be covered, preventing its primary functions.
[4][2] AP2 facilitates the assembly of clathrin lattices when endocytosis needs to occur, by aggregating together with other AP2 complexes, in their active conformation.
This is thought to occur by a secondary stabilization of the complex structure, which would allow partial activation, or access, to the respective pits.
[9] A family of proteins called muniscins are thought to be the primary allosteric activators of the AP2 adaptor complex,[10] due to their prevalence in AP2 associated pits and their inhibition resulting in the decrease in AP2 mediated endocytosis.
[15] In fact, it's seen to complex with phosphatidylinositol clathrin assembly lymphoid-myeloid leukemia (PICALM), which would serve as an important receptor group for microtubule-associated protein 1 light chain 3 (LC3).