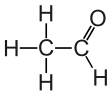
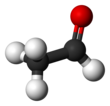
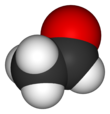
Acetaldehyde
[15] Acetaldehyde was first observed by the Swedish pharmacist/chemist Carl Wilhelm Scheele (1774);[16] it was then investigated by the French chemists Antoine François, comte de Fourcroy and Louis Nicolas Vauquelin (1800),[17] and the German chemists Johann Wolfgang Döbereiner (1821, 1822, 1832)[18] and Justus von Liebig (1835).
[25] This reaction is catalyzed by mercury(II) salts: The mechanism involves the intermediacy of vinyl alcohol, which tautomerizes to acetaldehyde.
[24] The enzyme Acetylene hydratase discovered in the strictly anaerobic bacterium Pelobacter acetylenicus can catalyze an analogous reaction without involving any compounds of mercury.
The hydroformylation of methanol with catalysts like cobalt, nickel, or iron salts also produces acetaldehyde, although this process is of no industrial importance.
[27] At room temperature, acetaldehyde (CH3CH=O) is more stable than vinyl alcohol (CH2=CHOH) by 42.7 kJ/mol:[28] Overall the keto-enol tautomerization occurs slowly but is catalyzed by acids.
[33] In one of the more spectacular addition reactions, formaldehyde in the presence of calcium hydroxide adds to MeCHO to give pentaerythritol, C(CH2OH)4 and formate.
[34] In a Strecker reaction, acetaldehyde condenses with cyanide and ammonia to give, after hydrolysis, the amino acid alanine.
[37] Three molecules of acetaldehyde condense to form "paraldehyde", a cyclic trimer containing C-O single bonds.
[41] In the brain, the enzyme catalase is primarily responsible for oxidizing ethanol to acetaldehyde, and alcohol dehydrogenase plays a minor role.
Demand has been impacted by changes in the production of plasticizer alcohols, which has shifted because n-butyraldehyde is less often produced from acetaldehyde, instead being generated by hydroformylation of propylene.
Likewise, acetic acid, once produced from acetaldehyde, is made predominantly by the lower-cost methanol carbonylation process.
However, Japan could emerge as a potential consumer for acetaldehyde in next five years due to newfound use in commercial production of butadiene.
[48][49] In 1988 the International Agency for Research on Cancer stated, "There is sufficient evidence for the carcinogenicity of acetaldehyde (the major metabolite of ethanol) in experimental animals.
[51] In addition, acetaldehyde is damaging to DNA[52] and causes abnormal muscle development as it binds to proteins.
[54] The second repair pathway requires replication fork convergence, breakage of the acetaldehyde crosslink, translesion synthesis by a Y-family DNA polymerase and homologous recombination.
[54] People with a genetic deficiency for the enzyme responsible for the conversion of acetaldehyde into acetic acid may have a greater risk of Alzheimer's disease.
"These results indicate that the ALDH2 deficiency is a risk factor for LOAD [late-onset Alzheimer's disease] ..."[55] A study of 818 heavy drinkers found that those exposed to more acetaldehyde than normal through a genetic variant of the gene encoding for ADH1C, ADH1C*1, are at greater risk of developing cancers of the upper gastrointestinal tract and liver.
[56] The drug disulfiram (Antabuse) inhibits acetaldehyde dehydrogenase, an enzyme that oxidizes the compound into acetic acid.
[citation needed] It has been concluded that volatile organic compounds (VOC) such as benzene, formaldehyde, acetaldehyde, toluene, and xylenes have to be considered priority pollutants with respect to their health effects.
[58] The use of acetaldehyde is widespread in different industries, and it may be released into waste water or the air during production, use, transportation and storage.
Sources of acetaldehyde include fuel combustion emissions from stationary internal combustion engines and power plants that burn fossil fuels, wood, or trash, oil and gas extraction, refineries, cement kilns, lumber and wood mills and paper mills.
[60] As a result, acetaldehyde is "one of the most frequently found air toxics with cancer risk greater than one in a million".
This finding emerged through the use of new chemical techniques that demonstrated the acetaldehyde present was causing DNA damage in laboratory settings.
Acetaldehyde, derived from mucosal or microbial oxidation of ethanol, tobacco smoke, and diet, appears to act as a cumulative carcinogen in the upper digestive tract of humans.
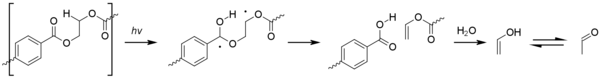
[67] Acetaldehyde can be produced by the photo-oxidation of polyethylene terephthalate (PET), via a Type II Norrish reaction.
[68] Although the levels produced by this process are minute acetaldehyde has an exceedingly low taste/odor threshold of around 20–40 ppb and can cause an off-taste in bottled water.
[70] Candida albicans in patients with potentially carcinogenic oral diseases has been shown to produce acetaldehyde in quantities sufficient to cause problems.