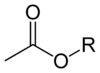
Acetate
"Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula C2H3O−2.
The pseudoelement symbol "Ac" is also sometimes encountered in chemical formulas as indicating the entire acetate ion (CH3CO−2).
[citation needed] It is not to be confused with the symbol of actinium, the first element of the actinide series; context guides disambiguation.
Although its systematic name is ethanoate (/ɪˈθænoʊ.eɪt/), the common acetate remains the preferred IUPAC name.
[6] Intraperitoneal injection of sodium acetate (20 or 60 mg per kg body mass) was found to induce headache in sensitized rats, and it has been proposed that acetate resulting from oxidation of ethanol is a major factor in causing hangovers.
Increased serum acetate levels lead to accumulation of adenosine in many tissues including the brain, and administration of the adenosine receptor antagonist caffeine to rats after ethanol was found to decrease nociceptive behavior.
This acetyl-CoA is then converted into acetate in E. coli, whilst producing ATP by substrate-level phosphorylation.
[10] acetyl-CoA + phosphate → acetyl-phosphate + CoA acetyl-phosphate + ADP → acetate + ATP Acetic acid can also undergo a dismutation reaction to produce methane and carbon dioxide:[11][12] This disproportionation reaction is catalysed by methanogen archaea in their fermentative metabolism.