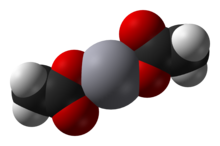
Mercury(II) acetate
Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å.
Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg.
This behavior is illustrated with phenol: The acetate group (OAc) that remains on mercury can be displaced by chloride:[5] The Hg2+ center binds to alkenes, inducing the addition of hydroxide and alkoxide.
For example, treatment of methyl acrylate with mercuric acetate in methanol gives an α-mercuri ester:[6] Exploiting the high affinity of mercury(II) for sulfur ligands, Hg(OAc)2 can be used as a reagent to deprotect thiol groups in organic synthesis.
Similarly Hg(OAc)2 has been used to convert thiocarbonate esters into dithiocarbonates: Mercury(II) acetate is used for oxymercuration reactions.