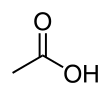
Acetic acid
Its production and subsequent industrial use poses health hazards to workers, including incidental skin damage and chronic respiratory injuries from inhalation.
Vinegar was known early in civilization as the natural result of exposure of beer and wine to air because acetic acid-producing bacteria are present globally.
The use of acetic acid in alchemy extends into the third century BC, when the Greek philosopher Theophrastus described how vinegar acted on metals to produce pigments useful in art, including white lead (lead carbonate) and verdigris, a green mixture of copper salts including copper(II) acetate.
Ancient Romans boiled soured wine to produce a highly sweet syrup called sapa.
[13] In the 16th-century German alchemist Andreas Libavius described the production of acetone from the dry distillation of lead acetate, ketonic decarboxylation.
[17] However, a lack of practical materials that could contain the corrosive reaction mixture at the high pressures needed (200 atm or more) discouraged commercialization of these routes.
The first commercial methanol carbonylation process, which used a cobalt catalyst, was developed by German chemical company BASF in 1963.
In the late 1990s, BP Chemicals commercialised the Cativa catalyst ([Ir(CO)2I2]−), which is promoted by iridium for greater efficiency.
Acetic acid has the distinction of being the first molecule discovered in the interstellar medium using solely radio interferometers; in all previous ISM molecular discoveries made in the millimetre and centimetre wavelength regimes, single dish radio telescopes were at least partly responsible for the detections.
These bacteria are found universally in foodstuffs, water, and soil, and acetic acid is produced naturally as fruits and other foods spoil.
Acetic acid is also a component of the vaginal lubrication of humans and other primates, where it appears to serve as a mild antibacterial agent.
Other processes are methyl formate isomerization, conversion of syngas to acetic acid, and gas phase oxidation of ethylene and ethanol.
As of 2003–2005, total worldwide production of virgin acetic acid[b] was estimated at 5 Mt/a (million tonnes per year), approximately half of which was produced in the United States.
[40] Light naphtha components are readily oxidized by oxygen or even air to give peroxides, which decompose to produce acetic acid according to the chemical equation, illustrated with butane: Such oxidations require metal catalyst, such as the naphthenate salts of manganese, cobalt, and chromium.
A similar process uses the same metal catalyst on silicotungstic acid and silica:[43] It is thought to be competitive with methanol carbonylation for smaller plants (100–250 kt/a), depending on the local price of ethylene.
Commonly used feeds include apple cider, wine, and fermented grain, malt, rice, or potato mashes.
The overall chemical reaction facilitated by these bacteria is: A dilute alcohol solution inoculated with Acetobacter and kept in a warm, airy place will become vinegar over the course of a few months.
The alcohol-containing feed is trickled into the top of the tower, and fresh air supplied from the bottom by either natural or forced convection.
[47] In this method, alcohol is fermented to vinegar in a continuously stirred tank, and oxygen is supplied by bubbling air through the solution.
[45] Species of anaerobic bacteria, including members of the genus Clostridium or Acetobacterium, can convert sugars to acetic acid directly without creating ethanol as an intermediate.
The overall chemical reaction conducted by these bacteria may be represented as: These acetogenic bacteria produce acetic acid from one-carbon compounds, including methanol, carbon monoxide, or a mixture of carbon dioxide and hydrogen: This ability of Clostridium to metabolize sugars directly, or to produce acetic acid from less costly inputs, suggests that these bacteria could produce acetic acid more efficiently than ethanol-oxidizers like Acetobacter.
[27] The reaction consists of ethylene and acetic acid with oxygen over a palladium catalyst, conducted in the gas phase.
In addition, ether acetates are used as solvents for nitrocellulose, acrylic lacquers, varnish removers, and wood stains.
[51] Glacial acetic acid is used in analytical chemistry for the estimation of weakly alkaline substances such as organic amides.
[55] Acetic acid is an effective antiseptic when used as a 1% solution, with broad spectrum of activity against streptococci, staphylococci, pseudomonas, enterococci and others.
[59] While diluted acetic acid is used in iontophoresis, no high quality evidence supports this treatment for rotator cuff disease.
Table vinegar tends to be more diluted (4% to 8% acetic acid), while commercial food pickling employs solutions that are more concentrated.
The OH group is the main site of reaction, as illustrated by the conversion of acetic acid to acetyl chloride.
Vapour concentrations of 1,000 ppm cause marked irritation of eyes, nose and upper respiratory tract and cannot be tolerated.
Exposure to 50 ppm or more is intolerable to most persons and results in intensive lacrimation and irritation of the eyes, nose, and throat, with pharyngeal oedema and chronic bronchitis.