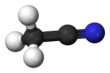
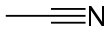Acetonitrile
With a dipole moment of 3.92 D,[8] acetonitrile dissolves a wide range of ionic and nonpolar compounds and is useful as a mobile phase in HPLC and LC–MS.
It is widely used in battery applications because of its relatively high dielectric constant and ability to dissolve electrolytes.
Acetonitrile plays a significant role as the dominant solvent used in oligonucleotide synthesis from nucleoside phosphoramidites.
[9] Acetonitrile is a common two-carbon building block in organic synthesis[10] of many useful chemicals, including acetamidine hydrochloride, thiamine, and 1-naphthaleneacetic acid.
[5] Acetonitrile has a free electron pair at the nitrogen atom, which can form many transition metal nitrile complexes.
Most is combusted to support the intended process but an estimated several thousand tons are retained for the above-mentioned applications.
Starting in October 2008, the worldwide supply of acetonitrile was low because Chinese production was shut down for the Olympics.
[20] The symptoms, which do not usually appear for several hours after the exposure, include breathing difficulties, slow pulse rate, nausea, and vomiting.
At least two cases have been reported of accidental poisoning of young children by acetonitrile-based nail polish remover, one of which was fatal.
[22] Acetone and ethyl acetate are often preferred as safer for domestic use, and acetonitrile has been banned in cosmetic products in the European Economic Area since March 2000.
[23] In common with other nitriles, acetonitrile can be metabolised in microsomes, especially in the liver, to produce hydrogen cyanide, as was first shown by Pozzani et al. in 1959.