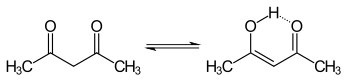
Acetylacetone
The equilibrium constant tends to be high in nonpolar solvents; when Kketo→enol is equal or greater than 1, the enol form is favoured.
IUPAC recommended pKa values for this equilibrium in aqueous solution at 25 °C are 8.99 ± 0.04 (I = 0), 8.83 ± 0.02 (I = 0.1 M NaClO4) and 9.00 ± 0.03 (I = 1.0 M NaClO4; I = Ionic strength).
The resulting dilithium species can then be alkylated at the carbon atom at the position 1.Acetylacetone is prepared industrially by the thermal rearrangement of isopropenyl acetate.
Acetone and acetic anhydride ((CH3C(O))2O) upon the addition of boron trifluoride (BF3) catalyst:[11] A second synthesis involves the base-catalyzed condensation (e.g., by sodium ethoxide CH3CH2O−Na+) of acetone and ethyl acetate, followed by acidification of the sodium acetylacetonate (e.g., by hydrogen chloride HCl):[11] Because of the ease of these syntheses, many analogues of acetylacetonates are known.
Acetylacetone is a versatile bifunctional precursor to heterocycles because both keto groups may undergo condensation.
Condensation with two aryl- or alkylamines gives NacNacs, wherein the oxygen atoms in acetylacetone are replaced by NR (R = aryl, alkyl).
In some cases the chelate effect is so strong that no added base is needed to form the complex.