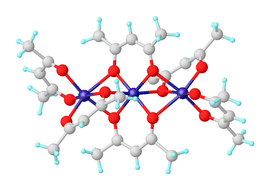
Nickel(II) bis(acetylacetonate)
Each nickel atom has tetragonally distorted octahedral geometry, caused by the difference in the length of the Ni–O bonds between the bridging and non-bridging oxygens.
[4] When bound to bulkier analogues of acetylacetonate ligand, steric hindrance favors formation of the mononickel derivatives.
[9] This complex can be dehydrated using a Dean–Stark trap by azeotropic distillation:[9] Upon heating Ni(acac)2(H2O)2 at 170–210 °C under reduced pressure (0.2–0.4 mmHg, 27–53 Pa), the anhydrous form sublimes and water is removed.
[10] Illustrative is the reaction with tetramethylethylenediamine (tmeda):[11] Ni(acac)2(H2O)2 reacts quickly in high yield at a methine positions, producing diamides from isocyanates.
[12][11] [Ni(acac)2]3 is a precursor for the deposition of a thin film of NiO on conductive glass substrates using sol-gel techniques.