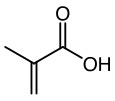
Methacrylic acid
This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor.
Another route to methacrylic acid starts with isobutylene, which obtainable by dehydration of tert-butanol.
[8] MAA occurs naturally in small amounts in the oil of Roman chamomile.
For commercial applications, MAA is polymerized using azobisisobutyronitrile as a thermally activated free-radical catalyst.
Esterifications are brought about by acid-catalyzed condensations with alcohols, alkylations with certain alkenes, and transesterifications.