Butadiene
Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles.
[11] This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum.
[12] In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a material with rubber-like properties.
This polymer was, however, found to be too soft to replace natural rubber in many applications, notably automobile tires.
[13] In 1929, Eduard Tschunker and Walter Bock, working for IG Farben in Germany, made a copolymer of styrene and butadiene that could be used in automobile tires.
Worldwide production quickly ensued, with butadiene being produced from grain alcohol in the Soviet Union and the United States, and from coal-derived acetylene in Germany.
The first such post-war commercial plant, producing 65,000 tons per year of butadiene, began operations in 1957 in Houston, Texas.
Today, butadiene from n-butane is commercially produced using the Houdry Catadiene process, which was developed during World War II.
[18] In other parts of the world, including South America, Eastern Europe, China, and India, butadiene is also produced from ethanol.
While not competitive with steam cracking for producing large volumes of butadiene, lower capital costs make production from ethanol a viable option for smaller-capacity plants.
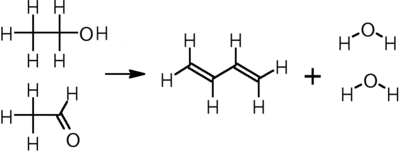
In the single-step process developed by Sergei Lebedev, ethanol is converted to butadiene, hydrogen, and water at 400–450 °C over any of a variety of metal oxide catalysts:[19] This process was the basis for the Soviet Union's synthetic rubber industry during and after World War II, and it remained in limited use in Russia and other parts of eastern Europe until the end of the 1970s.
In the other, two-step process, developed by the Russian emigre chemist Ivan Ostromislensky, ethanol is oxidized to acetaldehyde, which reacts with additional ethanol over a tantalum-promoted porous silica catalyst at 325–350 °C to yield butadiene:[19] This process was one of the three used in the United States to produce "government rubber" during World War II, although it is less economical than the butane or butene routes for the large volumes.
The USSRP constructed several plants in Baton Rouge and Lake Charles, Louisiana; Houston, Baytown, and Port Neches, Texas; and Torrance, California.
In the 1960s, a Houston company known as "Petro-Tex" patented a process to produce butadiene from normal butenes by oxidative dehydrogenation using a proprietary catalyst.
The conversion of butadiene to synthetic rubbers is called polymerization, a process by which small molecules (monomers) are linked to make large ones (polymers).
Butadiene is also useful in the synthesis of cycloalkanes and cycloalkenes, as it reacts with double and triple carbon-carbon bonds through Diels-Alder reactions.
This conformation is most stable because orbital overlap between double bonds is maximized, allowing for maximum conjugation, while steric effects are minimized.
In contrast, the s-cis conformation, in which the dihedral angle is 0°, with the pair of double bonds facing the same direction is approximately 16.5 kJ/mol (3.9 kcal/mol) higher in energy, due to steric hindrance.
[32][33] The American Conference of Governmental Industrial Hygienists (ACGIH) lists the chemical as a suspected carcinogen.
[33] The Natural Resource Defense Council (NRDC) lists some disease clusters that are suspected to be associated with this chemical.
[39] Animal data suggest that women have a higher sensitivity to possible carcinogenic effects of butadiene over men when exposed to the chemical.
There is also a lack of human data for the effects of butadiene on reproductive and development shown to occur in mice, but animal studies have shown breathing butadiene during pregnancy can increase the number of birth defects, and humans have the same hormone systems as animals.