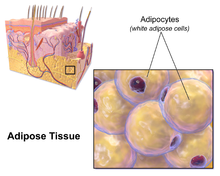
Adipose tissue
In the integumentary system, which includes the skin, it accumulates in the deepest level, the subcutaneous layer, providing insulation from heat and cold.
It may remain as a literal "apron of skin" if a severely obese person loses large amounts of fat (a common result of gastric bypass surgery).
[30] Epicardial adipose tissue (EAT) is a particular form of visceral fat deposited around the heart and found to be a metabolically active organ that generates various bioactive molecules, which might significantly affect cardiac function.
[31][32][33] Marked component differences have been observed in comparing EAT with subcutaneous fat, suggesting a location-specific impact of stored fatty acids on adipocyte function and metabolism.
[35] This subcutaneous fat is not related to many of the classic obesity-related pathologies, such as heart disease, cancer, and stroke, and some evidence even suggests it might be protective.
The most popular of these equations was formed by Durnin and Wormersley, who rigorously tested many types of skinfold, and, as a result, created two formulae to calculate the body density of both men and women.
The cause is likely a combination of genetic, environmental, and behavioral factors that are involved in excess energy intake and decreased physical activity.
[46] In the latter case, non-invasive weight loss interventions like diet or exercise can decrease ectopic fat (particularly in heart and liver) in overweight or obese children and adults.
[2] Human fat tissue contains from 61% to 94% lipids, with obese and lean individuals tending towards the high and low ends of this range, respectively.
Fat cells have an important physiological role in maintaining triglyceride and free fatty acid levels, as well as determining insulin resistance.
This explains to a large degree why central obesity is a marker of impaired glucose tolerance and is an independent risk factor for cardiovascular disease (even in the absence of diabetes mellitus and hypertension).
This suggests a possible cause-and-effect link between the two, wherein stress promotes the accumulation of visceral fat, which in turn causes hormonal and metabolic changes that contribute to heart disease and other health problems.
[54][55] These depot-dependent features include proliferation rate, immunophenotype, differentiation potential, gene expression, as well as sensitivity to hypoxic culture conditions.
Techniques to manipulate the differentiation of "brown fat" could become a mechanism for weight loss therapy in the future, encouraging the growth of tissue with this specialized metabolism without inducing it in other organs.
The calorie-burning capacity of brown and beige fat has been extensively studied as research efforts focus on therapies targeted to treat obesity and diabetes.
However, the use of such drugs has proven largely unsuccessful due to several challenges, including varying species receptor specificity and poor oral bioavailability.
[76][77][78] The list of molecules that influence browning has grown in direct proportion to the popularity of this topic and is constantly evolving as more knowledge is acquired.
[79] FGF21, a hormone secreted mainly by the liver, has garnered a great deal of interest after being identified as a potent stimulator of glucose uptake and a browning regulator through its effects on PGC-1α.
[70] It is increased in BAT during cold exposure and is thought to aid in resistance to diet-induced obesity[80] FGF21 may also be secreted in response to exercise and a low protein diet, although the latter has not been thoroughly investigated.
In mice, it was found that beiging can occur through the production of methionine-enkephalin peptides by type 2 innate lymphoid cells in response to interleukin 33.
[83] Due to the complex nature of adipose tissue and a growing list of browning regulatory molecules, great potential exists for the use of bioinformatics tools to improve study within this field.
Studies of WAT browning have greatly benefited from advances in these techniques, as beige fat is rapidly gaining popularity as a therapeutic target for the treatment of obesity and diabetes.
DNA microarray is a bioinformatics tool used to quantify expression levels of various genes simultaneously, and has been used extensively in the study of adipose tissue.
One such study used microarray analysis in conjunction with Ingenuity IPA software to look at changes in WAT and BAT gene expression when mice were exposed to temperatures of 28 and 6 °C.
Mössenböck et al. also used microarray analysis to demonstrate that insulin deficiency inhibits the differentiation of beige adipocytes but does not disturb their capacity for browning.
Incorporating RNA-Seq into browning studies is of great value, as it offers better specificity, sensitivity, and a more comprehensive overview of gene expression than other methods.
Chromatin immunoprecipitation with sequencing (ChIP-seq) is a method used to identify protein binding sites on DNA and assess histone modifications.
This tool has enabled examination of epigenetic regulation of browning and helps elucidate the mechanisms by which protein-DNA interactions stimulate the differentiation of beige adipocytes.
[87][88] In 1995, Jeffrey Friedman, in his residency at the Rockefeller University, together with Rudolph Leibel, Douglas Coleman et al. discovered the protein leptin that the genetically obese mouse lacked.
Within the fat (adipose) tissue of CCR2 deficient mice, there is an increased number of eosinophils, greater alternative Macrophage activation, and a propensity towards type 2 cytokine expression.