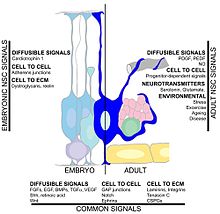
Adult neurogenesis
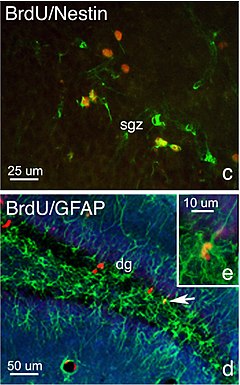
In rodents, many of the newborn dentate gyrus neurons die shortly after they are born,[4] but a number of them become functionally integrated into the surrounding brain tissue.
[10][11][12] Adult neurogenesis in rodents is reported to play a role in learning and memory, emotion, stress, depression, response to injury, and other conditions.
Emerging evidence suggests that neural microvascular pericytes, under instruction from resident glial cells, are reprogrammed into interneurons and enrich local neuronal microcircuits.
This model reproduces asexually producing a complete and fully functioning nervous system after division allowing for consistent examination of neurogenesis.
Though the axolotl has made its place in biomedical research in terms of limb regeneration,[19][20] the model organism has displayed a robust ability to generate new neurons following damage.
The zebrafish is a rapidly developing organism that is relatively inexpensive to maintain, while providing the field ease of genetic manipulation and a complex nervous system.
Rodents display a wide range of neural circuits responsible for complex behaviors making them ideal for studies of dendritic pruning and axonal shearing.
To some extent, adult neurogenesis in rodents may be induced by selective disruption of Notch signalling in astrocytes:[32] this produces novel neurons which functionally integrate into the striatal circuit.
[33] Adult neurogenesis in the subventricular zone and dentate gyrus of rodents generates oxidative stress and production of reactive oxygen species that can damage both DNA and lipids.
[40][circular reference] Black-capped chickadees are a well-known model species in the field of neuroscience for their neural mechanisms in song vocalization, plasticity, and memory.
Seasonal changes in hippocampal densities have been described since 1994[41] where neuronal survival peaks during the fall (October),[41] measured by thymidine (see tracking neurogenesis below) labeled cells, weeks after injection.
Taken all together, adult neurogenesis in the hippocampus of black-capped chickadees suggest a selective mechanisms for neuronal survival in direct correlation with seasonal food caching behavior.
[48] Reduced neurogenesis in captive birds may be caused by stress, lack of exercise, diminished social interaction, and limited caching opportunities.
It has recently become more common to use recombinant viruses to insert the genetic information encoding specific markers (usually protein fluorophores such as GFP) that are only expressed in cells of a certain kind.
[60][61] The changes in learning and memory seen in the studies mentioned previously are thought to be related to the role of adult neurogenesis in regulating pattern separation.
A decreased ability in reducing interference could lead to greater difficulty in forming and retaining new memories,[13] although it's hard to discriminate between effects of neurogenesis in learning and pattern separation due to limitations in the interpretation of behavioral results.
[72] Given that the rate of the neurogenesis does not change substantially during the adulthood, it has been proposed that unique episodic memories can be created by simply relying on the increased capacity of the young neurons of a particular age to establish stable new synapses with peers representing the unique features of an event to be memorized [73] Experiments aimed at ablating neurogenesis have proven inconclusive, but several studies have proposed neurogenic-dependence in some types of learning,[74] and others seeing no effect.
[81] Eph receptors and ephrin signaling have been shown to regulate adult neurogenesis in the hippocampus and have been studied as potential targets to treat some symptoms of AD.
Some neuroscientists have expressed skepticism that neurogenesis is functionally significant, given that a tiny number of nascent neurons are actually integrated into existing neural circuitry.
However, a recent study used the irradiation of nascent hippocampal neurons in non-human primates (NHP) to demonstrate that neurogenesis is required for antidepressant efficacy.
[94][95] In a pioneer study, scientists demonstrated that the behavioral benefits of antidepressant administration in mice is reversed when neurogenesis is prevented with x-irradiation techniques.
[101] In recent years, scientists have provided evidence for the existence of neural stem cells with the potential to produce new neurons, particularly of a dopaminergic phenotype, in the adult mammalian brain.
Hormonal interventions, such as progesterone, estrogen, and allopregnanolone have been examined heavily in recent decades as possible neuroprotective agents following traumatic brain injuries to reduce the inflammation response stunt neuronal death.
[116] Scientists have shown that physical activity in the form of voluntary exercise results in an increase in the number of newborn neurons in the hippocampus of mice and rats.
[121] Studies have shown that a synthetic drug resembling THC, the main psychoactive ingredient in marijuana, provides some protection against brain inflammation, which might result in better memory at an older age.
[122] Nonetheless, a study directed at Rutgers University demonstrated how synchronization of action potentials in the hippocampus of rats was altered after THC administration.
[124] A greater CBD to THC ratio in hair analyses of cannabis users correlates with protection against gray matter reduction in the right hippocampus.
[125] CBD has also been observed to attenuate the deficits in prose recall and visuo-spatial associative memory of those currently under the influence of cannabis,[126][127] implying neuroprotective effects against heavy THC exposure.
DNA methylation is critical in the fate-determination of adult neural stem cells in the subventricular zone for post-natal neurogenesis through the regulation of neuronic genes such as Dlx2, Neurog2, and Sp8.
[144] In 1969, Joseph Altman discovered and named the rostral migratory stream as the source of adult generated granule cell neurons in the olfactory bulb.