Humoral immunity
The study of the molecular and cellular components that form the immune system, including their function and interaction, is the central science of immunology.
[2] In 1890, Buchner described alexins as "protective substances" that exist in the blood serum and other bodily fluids and are capable of killing microorganisms.
Alexins, later redefined as "complements" by Paul Ehrlich, were shown to be the soluble components of the innate response that leads to a combination of cellular and humoral immunity.
[2] Ehrlich, with his colleague von Behring, went on to develop the diphtheria antitoxin, which became the first major success of modern immunotherapy.
By binding their specific antigens, antibodies can cause agglutination and precipitation of antibody-antigen products, prime for phagocytosis by macrophages and other cells, block viral receptors, and stimulate other immune responses, such as the complement pathway.
The major complication is that hemoglobin released by the destruction of red blood cells can cause acute kidney failure.
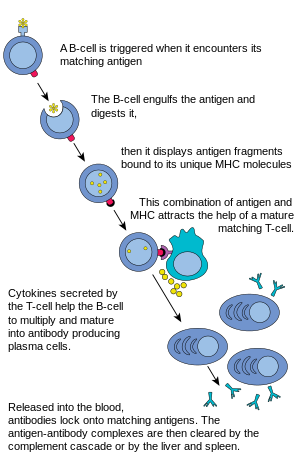
The mature B cells then migrate from the bone marrow to the lymph nodes or other lymphatic organs, where they begin to encounter pathogens.
[8] B cells can be activated through certain microbial agents without the help of T-cells and have the ability to work directly with antigens to provide responses to pathogens present.
This will either interfere with the chemical interaction between host and foreign cells, or they may form bridges between their antigenic sites hindering their proper functioning.
Activation of this system leads to cytolysis, chemotaxis, opsonization, immune clearance, and inflammation, as well as the marking of pathogens for phagocytosis.
This differs from the mannose-binding lectin pathway, which is initiated by bacterial carbohydrate motifs, such as mannose, found on the surface of bacterium.
In all three pathways, once C3 convertase is synthesized, complements are cleaved into subunits which either form a structure called the membrane attack complex (MAC) on the bacterial cell wall to destroy the bacteria [11] or act as cytokines and chemokines, amplifying the immune response.