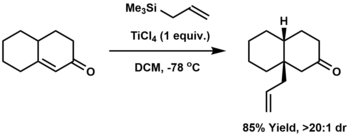Allyl group
In organic chemistry, an allyl group is a substituent with the structural formula −CH2−HC=CH2.
In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl".
Benzylic and allylic are related in terms of structure, bond strength, and reactivity.
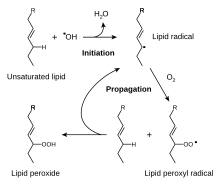
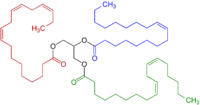
[5] One practical consequence of their high reactivity is that polyunsaturated fatty acids have poor shelf life owing to their tendency toward autoxidation, leading, in the case of edibles, to rancidification.
All feature three contiguous sp²-hybridized carbon centers and all derive stability from resonance.
[6] Each species can be presented by two resonance structures with the charge or unpaired electron distributed at both 1,3 positions.
The sulfur vulcanization or various rubbers exploits the conversion of allylic CH2 groups into CH−Sx−CH crosslinks.
[13] One commercial application of allylic oxidation is the synthesis of nootkatone, the fragrance of grapefruit, from valencene, a more abundantly available sesquiterpenoid:[14] In the synthesis of some fine chemicals, selenium dioxide is used to convert alkenes to allylic alcohols:[15] where R, R', R" may be alkyl or aryl substituents.
[16] Many substituents can be attached to the allyl group to give stable compounds.