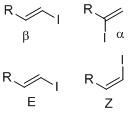
Vinyl iodide functional group
Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis.
In SN2 reactions, back-attack is difficult because of steric clash of R groups on carbon adjacent to electrophilic center (see figure 1a).
[2] In addition, the lone pair on iodide donates into the ╥* of the alkene, which reduces electrophilic character on the carbon as a result of decreased positive charge.
Also, this stereoelectronic effect strengthens the C-I bond, thus making removal of the iodide difficult (see figure 1b).
[6] However, there is evidence in literature, in which a propargyl alcohol's alkyne was reduced in presence of a vinyl iodide using hydrogen over Pd/CaCO3 or Crabtree's catalyst.
[8] The scope of this synthetic method is limited since it requires higher temperatures and longer reaction time, which affects functional group tolerance.
However, vinyl iodide with electron withdrawing group can enhance rate of exchange(see Scheme 1b).
Vinyl iodides with well-defined geometry (regiochemistry and stereochemistry) are important in synthesis since many natural products and drugs that have specific structure and dimension.
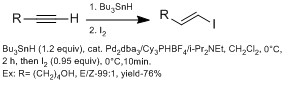
In synthesis, it is useful to introduce vinyl iodide at various positions to be set up for a coupling reaction at the next synthetic step.
The common and simplest approach to make vinyl iodide is addition of one equivalent HI to alkyne.
However, Hoveyda group have demonstrated using nickel-based catalyst (Ni(dppp)Cl2), DIBAL-H with N-iodosuccinimide (NIS), selectively favor α-vinyl iodide with little to no byproducts.
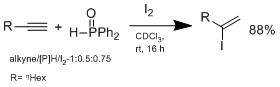
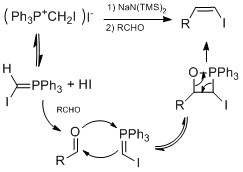
Another method doesn't involve hydrometalation but hydroiodation with I2/hydrophosphine binary system, which was developed by Ogawa's group.
In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene.
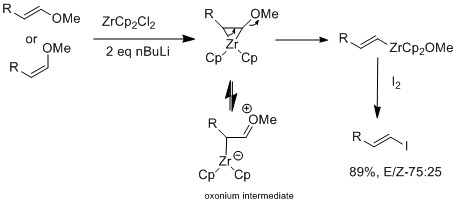
The difference of results between halogen exchange and E-vinyl ether reaction is that only when there is a presence of an oxonium intermediate, is isomerization observed.
The Whiting group, however, noticed that Brown's method was not applicable to more sterically hindered boronic esters (no reaction).
The advantages of iododesilylation are that it avoids toxic tin reagent and intermediate vinyl silyl are stable, nontoxic and easily handled and stored.
If the R group is small, the solvent acetonitrile can participate in the reaction leading to inversion of the olefin's geometry.
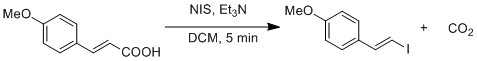
In addition, they observed accelerated reaction rate because HFIP activate NIS by hydrogen bonding.
[26] Another method is the Takai olefination which uses iodoform and chromium(II) chloride to make vinyl iodide from aldehyde with high stereoselectivity for E geometry.