IUPAC nomenclature of organic chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended[1][2] by the International Union of Pure and Applied Chemistry (IUPAC).
[3] Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created.
For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over.
In chemistry, a number of prefixes, suffixes and infixes are used to describe the type and position of the functional groups in the compound.
Straight-chain alkanes take the suffix "-ane" and are prefixed depending on the number of carbon atoms in the chain, following standard rules.
They are prefixed with a number indicating the carbon the group is attached to, counting from the end of the alkane chain.
For example, (CH3)2CHCH3, commonly known as isobutane, is treated as a propane chain with a methyl group bonded to the middle (2) carbon, and given the systematic name 2-methylpropane.
However, although the name 2-methylpropane could be used, it is easier and more logical to call it simply methylpropane – the methyl group could not possibly occur on any of the other carbon atoms (that would lengthen the chain and result in butane, not propane) and therefore the use of the number "2" is unnecessary.
The longest possible main alkane chain is used; therefore 3-ethyl-4-methylhexane instead of 2,3-diethylpentane, even though these describe equivalent structures.
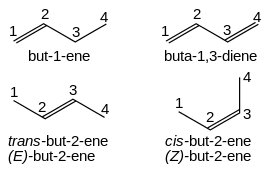
Simple cis and trans isomers may be indicated with a prefixed cis- or trans-: cis-but-2-ene, trans-but-2-ene.
It is IUPAC convention to describe all alkenes using absolute descriptors of Z- (same side) and E- (opposite) with the Cahn–Ingold–Prelog priority rules (see also E–Z notation).
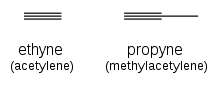
Alkynes are named using the same system, with the suffix "-yne" indicating a triple bond: ethyne (acetylene), propyne (methylacetylene).
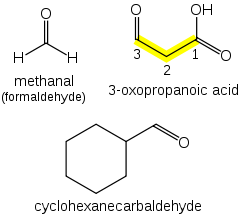
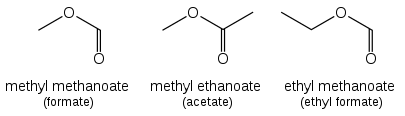
If a prefix form is required, "oxo-" is used (as for ketones), with the position number indicating the end of a chain: CHOCH2COOH is 3-oxopropanoic acid.
If the carbon in the carbonyl group cannot be included in the attached chain (for instance in the case of cyclic aldehydes), the prefix "formyl-" or the suffix "-carbaldehyde" is used: C6H11CHO is cyclohexanecarbaldehyde.
If an aldehyde is attached to a benzene and is the main functional group, the suffix becomes benzaldehyde.
As with aldehydes, the carboxyl functional group must take the "1" position on the main chain and so the locant need not be stated.
In the latter case, the carbon atoms in the carboxyl groups do not count as being part of the main chain, a rule that also applies to the prefix form "carboxy-".
Salts of carboxylic acids are named following the usual cation-then-anion conventions used for ionic compounds in both IUPAC and common nomenclature systems.
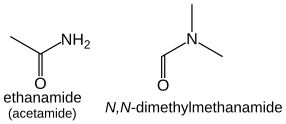
For secondary amines (of the form R−NH−R), the longest carbon chain attached to the nitrogen atom becomes the primary name of the amine; the other chain is prefixed as an alkyl group with location prefix given as an italic N: CH3NHCH2CH3 is N-methylethanamine.
Amides that have additional substituents on the nitrogen are treated similarly to the case of amines: they are ordered alphabetically with the location prefix N: HCON(CH3)2 is N,N-dimethylmethanamide, CH3CON(CH3)2 is N,N-dimethylethanamide.Nitriles (R−C≡N) are named by adding the suffix "-nitrile" to the longest hydrocarbon chain (including the carbon of the cyano group).
For example, the three isomers of xylene CH3C6H4CH3, commonly the ortho-, meta-, and para- forms, are 1,2-dimethylbenzene, 1,3-dimethylbenzene, and 1,4-dimethylbenzene.
The cyclic structures can also be treated as functional groups themselves, in which case they take the prefix "cycloalkyl-" (e.g. "cyclohexyl-") or for benzene, "phenyl-".
The IUPAC nomenclature scheme becomes rapidly more elaborate for more complex cyclic structures, with notation for compounds containing conjoined rings, and many common names such as phenol being accepted as base names for compounds derived from them.
Prefixed substituents are ordered alphabetically (excluding any modifiers such as di-, tri-, etc.
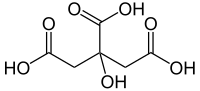
If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically (thus ethane-1,2-diol, not ethane-2,1-diol.)
*Note: These suffixes, in which the carbon atom is counted as part of the preceding chain, are the most commonly used.
[clarification needed] Common nomenclature uses the older names for some organic compounds instead of using the prefixes for the carbon skeleton above.
However, many organic cations are obtained by substituting another element or some functional group for a hydrogen.
If many substitutions by the same functional group occur, then the number is indicated by prefixing with "di-", "tri-" as with halogenation.