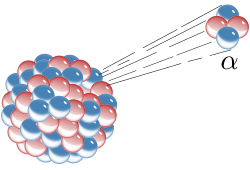
Alpha particle
Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom 42He.
They are a highly ionizing form of particle radiation, with low penetration depth (stopped by a few centimetres of air, or by the skin).
The term "alpha particle" was coined by Ernest Rutherford in reporting his studies of the properties of uranium radiation.
After five years of additional experimental work, Rutherford and Hans Geiger determined that "the alpha particle, after it has lost its positive charge, is a Helium atom".
[7][8][9]: 61 Alpha radiation consists of particles equivalent to doubly-ionized helium nuclei (He2+) which can gain electrons from passing through matter.
Alpha particles are commonly emitted by all of the larger radioactive nuclei such as uranium, thorium, actinium, and radium, as well as the transuranic elements.
The alpha decay sometimes leaves the parent nucleus in an excited state; the emission of a gamma ray then removes the excess energy.
In addition, extremely high energy helium nuclei sometimes referred to as alpha particles make up about 10 to 12% of cosmic rays.
[12] Because of their charge and large mass, alpha particles are easily absorbed by materials, and they can travel only a few centimetres in air.
Due to the short range of absorption and inability to penetrate the outer layers of skin, alpha particles are not, in general, dangerous to life unless the source is ingested or inhaled.
[9]: 49 Marie Curie showed that this phenomenon, which she called "radioactivity", was not unique to uranium and a consequence of individual atoms.
[18] In 1900, Marie Curie noticed that the absorption coefficient of alpha rays seemed to increase the thicker the barrier she placed in their path.
This suggested that alpha radiation is not a form of light but made of particles that lose kinetic energy as they pass through barriers.
His explanation was that as alpha particles are emitted by underground radioactive elements, they become trapped in the rock strata and acquire electrons, becoming helium atoms.
[9]: 61 In 1911, Rutherford used alpha particle scattering data to argue that the positive charge of an atom is concentrated in a tiny nucleus.
While anti-matter equivalents for helium-3 have been known since 1970, it took until 2010 for members of the international STAR collaboration using the Relativistic Heavy Ion Collider at the U.S. Department of Energy's Brookhaven National Laboratory to detect the antimatter partner of the helium-4 nucleus.
This time the gold ions moving at nearly the speed of light and colliding head on to produce the antiparticle, also dubbed "anti-alpha" particle.
Radium-223 (as radium-223 dichloride) can be infused into a cancer patient's veins, after which it migrates to parts of the bone where there is rapid turnover of cells due to the presence of metastasized tumors.
However, radium-224's daughter atoms can diffuse up to 2–3 mm in the tissue, thus creating a "kill region" with enough radiation to potentially destroy an entire tumor, if the seeds are placed appropriately.
[33] Radium-224's half-life is short enough at 3.6 days to produce a rapid clinical effect while avoiding the risk of radiation damage due to overexposure.
At the same time, the half-life is long enough to allow for handling and shipping the seeds to a cancer treatment center at any location across the globe.
Targeted alpha therapy for solid tumors involves attaching an alpha-particle-emitting radionuclide to a tumor-targeting molecule such as an antibody, that can be delivered by intravenous administration to a cancer patient.
The discovery led to strict control of radioactive elements in the packaging of semiconductor materials, and the problem is largely considered to be solved.