Cloud chamber
Cloud chambers were invented in the early 1900s by the Scottish physicist Charles Thomson Rees Wilson.
The Discovery of the kaon by George Rochester and Clifford Charles Butler in 1947 was made using a cloud chamber as the detector.
[2] Charles Thomson Rees Wilson (1869–1959), a Scottish physicist, is credited with inventing the cloud chamber.
Inspired by sightings of the Brocken spectre while working on the summit of Ben Nevis in 1894, he began to develop expansion chambers for studying cloud formation and optical phenomena in moist air.
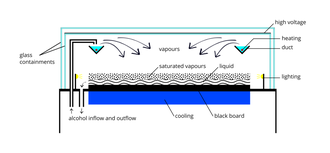
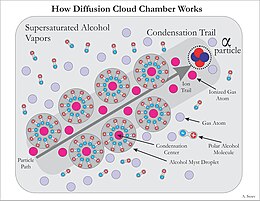
This occurs because alcohol and water molecules are polar, resulting in a net attractive force toward a nearby free charge (See Fig.
The ion trail left by the radioactive particles provides an optimal trigger for condensation and cloud formation.
This sensitive volume is increased in height by employing a steep temperature gradient, and stable conditions.
This method was also used to prove the existence of the Positron in 1932, in accordance with Paul Dirac's theoretical proof, published in 1928.
[8] The bubble chamber was invented by Donald A. Glaser of the United States in 1952, and for this, he was awarded the Nobel Prize in Physics in 1960.
The bubble chamber similarly reveals the tracks of subatomic particles, but inverts the principle of the cloud chamber to detect them as trails of bubbles in a superheated liquid, usually liquid hydrogen, rather than as trails of drops in a supercritical vapor.
Energetic charged particles cause ionization of the gas along the path of the particle in the same way as in the Wilson cloud chamber, but in this case the ambient electric fields are high enough to precipitate full-scale gas breakdown in the form of sparks at the position of the initial ionization.
The presence and location of these sparks is then registered electrically, and the information is stored for later analysis, such as by a digital computer.