Aminoaldehydes and aminoketones
[1] Examples include cathinones, methadone, molindone, pimeclone, ferruginine, and tropinone.
Aminoketones containing secondary amines are typically stable when the ketone is located on a ring, e.g. 4-piperidinone, triacetonamine, acridone Most members of this class are unstable towards self-condensation, however some important examples do exist as intermediates in biosynthetic pathways e.g. glutamate-1-semialdehyde.
The acyclic forms of certain amino sugars also qualify, for instance vancosamine.
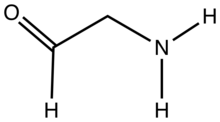
Aminoacetaldehyde, the simplest member of this subclass, is highly reactive toward self-condensation.
It is unstable under normal laboratory conditions, but the hydrochloride [CH3C(O)CH2NH3]Cl is readily isolable.