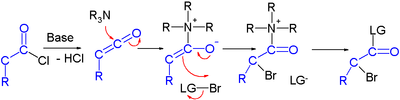
α-Halo ketone
In the Nierenstein reaction an acyl chloride reacts with diazomethane Efforts are reported in asymmetric synthesis of halo carbonyls through organocatalysis.
Illustrative of their alkylating activity are reactions with potassium iodide in acetone, chloroacetone reacts faster than 1-chloropropane by a factor of 36,000.
Due to the presence of two electron withdrawing groups (carbonyl and halide), the α-hydrogen is acidic.
This property is exploited in the Favorskii rearrangement, where base abstracts first an acidic α-hydrogen and the resulting carbanion then displaces the halogen.
[4] Halo ketones take part in several reaction types, especially since they are bifunctional, with two electrophilic sites (α-carbon and carbonyl carbon).