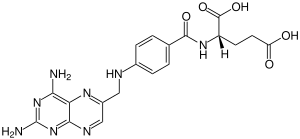
Aminopterin
[2][3] Aminopterin was later marketed by Lederle Laboratories (Pearl River, New York) in the United States from 1953 to 1964 for the indication of pediatric leukemia.
During the period Aminopterin was marketed, the agent was used off-label to safely treat over 4,000 patients with psoriasis in the United States, producing dramatic clearing of lesions.
[11] Aminopterin is widely used in selection media (such as HAT medium) for cell culture, particularly in the development of hybridomas, which secrete monoclonal antibodies.
[13] The link to aminopterin was confirmed by New York State Agriculture Commissioner Patrick Hooker and Dr. Donald Smith, Dean of Cornell University's College of Veterinary Medicine, in a statement released on the same day.
[20][21][22][23] Leucovorin rescue is a therapeutic maneuver intentionally employed with antifolates to achieve tumoricidal drug concentrations that would otherwise be lethal to the patient.
[22] Additionally, to prevent reversible aminopterin-mediated nephrotoxicity manifesting as increases in serum creatinine and which further delays drug elimination, urinary alkalinization with NaHCO3 and volume expansion should be considered in cases of massive aminopterin overdosage, particularly those involving greater than 100 mg AMT in an average 70 kg adult human.
Consistent with the known enterohepatic cycling of the related antifolate methotrexate, oral activated charcoal, and saline cathartic or sorbitol may promote excretion if an overdose of aminopterin is suspected.
The vitamin folic acid is an oxidized precursor to reduced folates that is upstream of the blockade at dihydrofolate reductase, and compared to leucovorin is recognized as a very weak antidote to the toxic effects of antifolates that is inappropriate for use in cases of acute intoxication.
[25][26] The temporal relationship between folic acid administration and rescue has been interpreted as the necessary period of time required for the vitamin to be converted in vivo to reduced forms.