Ammonia borane
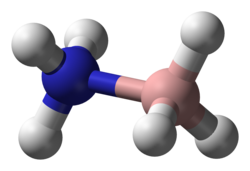
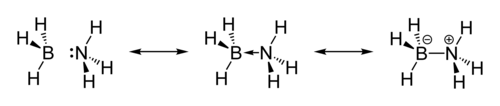
Ammonia borane (also systematically named ammoniotrihydroborate[citation needed]), also called borazane, is the chemical compound with the formula H3NBH3.
[10][11] The original crystallographic analysis of this compound reversed the assignments of B and N. The updated structure was arrived at with improved data using the technique of neutron diffraction that allowed the hydrogen atoms to be located with greater precision.
Ammonia borane has been suggested as a storage medium for hydrogen, e.g. for when the gas is used to fuel motor vehicles.
It can be made to release hydrogen on heating, being polymerized first to (NH2BH2)n, then to (NHBH)n,[15] which ultimately decomposes to boron nitride (BN) at temperatures above 1000 °C.
Borane tert-butylamine complex is prepared by the reaction of sodium borohydride with t-butylammonium chloride.

![Part of the crystal structure of ammonia borane[10]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/93/Ammonia-borane-xtal-3D-balls.png/300px-Ammonia-borane-xtal-3D-balls.png)