Boric acid
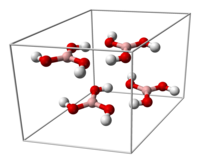
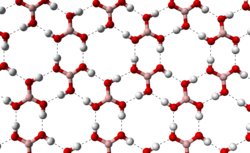
[6] The triclinic form of boric acid consists of layers of B(OH)3 molecules held together by hydrogen bonds with an O...O separation of 272 pm.
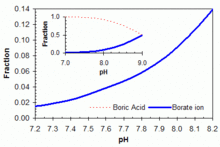
For example, the reaction with mannitol H(HCOH)6H, whose two middle hydroxyls are in cis orientation, can be written as Giving the overall reaction The stability of these mannitoborate ester anions shifts the equilibrium of the right and thus increases the acidity of the solution by 5 orders of magnitude compared to that of pure boric oxide, lowering the pKa from 9 to below 4 for sufficient concentration of mannitol.
[7] Based on mammalian median lethal dose (LD50) rating of 2,660 mg/kg body mass, boric acid is only poisonous if taken internally or inhaled in large quantities.
The Fourteenth Edition of the Merck Index indicates that the LD50 of boric acid is 5.14 g/kg for oral dosages given to rats, and that 5 to 20 g/kg has produced death in adult humans.
According to the Agency for Toxic Substances and Disease Registry, "The minimal lethal dose of ingested boron (as boric acid) was reported to be 2–3 g in infants, 5–6 g in children, and 15–20 g in adults.
"[23] Human studies in three borate exposure rich comparison groups (U.S. Borax mine and production facility workers, Chinese boron workers, Turkish residents living near boron rich regions) produced no indicators of developmental toxicity in blood and semen tests.
[25] According to the CLH report for boric acid published by the Bureau for Chemical Substances Lodz, Poland, boric acid in high doses shows significant developmental toxicity and teratogenicity in rabbit, rat, and mouse fetuses, as well as cardiovascular defects, skeletal variations, and mild kidney lesions.
[24] As a consequence in the 30th ATP to EU directive 67/548/EEC of August 2008, the European Commission decided to amend its classification as reprotoxic category 2 and to apply the risk phrases R60 (may impair fertility) and R61 (may cause harm to the unborn child).
[26][27][28][29][30] At a 2010 European Diagnostics Manufacturing Association (EDMA) Meeting, several new additions to the substance of very high concern (SVHC) candidate list in relation to the Registration, Evaluation, Authorisation and Restriction of Chemicals Regulations 2007 (REACH) were discussed.
Following the registration and review completed as part of REACH, the classification of boric acid CAS 10043-35-3 / 11113-50-1 is listed from 1 December 2010 is H360FD (May damage fertility.
Textile fiberglass is used to reinforce plastics in applications that range from boats, to industrial piping to computer circuit boards.
[33] In the jewelry industry, boric acid is often used in combination with denatured alcohol to reduce surface oxidation and formation of firescale on metals during annealing and soldering operations.
[40] Boric acid is also present in the list of chemical additives used for hydraulic fracturing (fracking) in the Marcellus Shale in Pennsylvania.
[41] It is often used in conjunction with guar gum as cross-linking and gelling agent for controlling the viscosity and the rheology of the fracking fluid injected at high pressure in the well.
It is important to control the fluid viscosity for keeping in suspension on long transport distances the grains of the propping agents aimed at maintaining the cracks in the shales sufficiently open to facilitate the gas extraction after the hydraulic pressure is relieved.
[55] Boric acid solutions used as an eye wash or on abraded skin are known to be toxic, particularly to infants, especially after repeated use; this is because of its slow elimination rate.
This process defeats the extreme toxicity of hydrofluoric acid, particularly its ability to sequester ionic calcium from blood serum which can lead to cardiac arrest and bone decomposition; such an event can occur from just minor skin contact with HF.
[57][failed verification] Boric acid was first registered in the US as an insecticide in 1948 for control of cockroaches, termites, fire ants, fleas, silverfish, and many other insects.
Boric acid also has the reputation as "the gift that keeps on killing" in that cockroaches that cross over lightly dusted areas do not die immediately, but that the effect is like shards of glass cutting them apart.
[citation needed] Boric acid in equilibrium with its conjugate base the borate ion is widely used (in the concentration range 50–100 ppm boron equivalents) as a primary or adjunct pH buffer system in swimming pools.
The use of boric acid in this concentration range does not allow any reduction in free HOCl concentration needed for pool sanitation, but it may add marginally to the photo-protective effects of cyanuric acid and confer other benefits through anti-corrosive activity or perceived water softness, depending on overall pool solute composition.
[62] Colloidal suspensions of nanoparticles of boric acid dissolved in petroleum or vegetable oil can form a remarkable lubricant on ceramic or metal surfaces[63] with a coefficient of sliding friction that decreases with increasing pressure to a value ranging from 0.10 to 0.02.
In bulk-scale, an inverse relationship exists between friction coefficient and Hertzian contact pressure induced by applied load.
Boric acid was dumped over Reactor 4 of the Chernobyl nuclear power plant after its meltdown to prevent another reaction from occurring.