Angelicin
The earliest known record dates back to 3000 BC when ancient Egyptians applied the oil and sap of local Apiaceae species exposing their skin to sunlight to cure vitiligo.
However, most of them turned out to be unpalatable and toxic such as Angelica archangelica due to the ability to irritate skin and damage internal organs.
This Latin name originated in medieval Europe where this plant was also used as a universal treatment to many types of disease not mentioning the bubonic plague.
At this time, people believed that the plant could prevent the soul from being taken over by sorcery, curse and evil spirit (add reference).
[8] There were multiple studies on the toxicity of angelicin one of which showed that the compound elicits chromosomal damage in hamster cells exposed to 320-380 nm UV light.
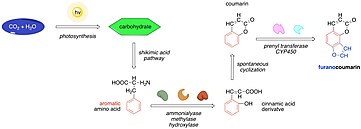
The newly formed trans-dihydrocinnamic acid undergoes a photochemical isomerization to a cis isomer which spontaneously lactonizes to yield umbeliferone.
Now, it is increasingly recognized that plants devised the pathway leading to angelicin as an alternative defense mechanism.
Acetoxy group can be introduced into hydroxyl of 7-hydroxy-8-iodocoumarin, which is used to create vaginol or vaginidiol with an isopropyl Grignard reagent and commercially available epoxy aldehydes.
[15] The compound can be isolated from natural sources, albeit this affords a low yield due to the prevalence of other furanocoumarins.
The popular technique is air drying the aerial parts and ground roots of plant followed by n-hexane extraction and column chromatography over silica gel.
[19] In PUVA, angelicin is less popular than psoralen, although both furanocoumarins are photosensitizing and used in couple with long-wave UV irradiation.
According to the mechanism, long-range UV light triggers angelicin to bind to the pyrimidine bases of DNA in the same manner as psoralen.
[21] It was shown that angelicin exhibits a multifaceted effect on various biomolecules which stem from the compound's structure and photoreactivity.
[22] However, the rest of the angelicin's aromatic system cannot react with the pyrimidine of complementary strand owing to the unfavorable alignment of reactive double bonds.
[31][32] Upon irradiation with UV light of longer wavelength, angelicin forms DNA monoadducts which can cause skin cancer.
[30][33] In mammalian cell cultures, angelicin showed mutagenic and cytotoxic effects while playing a role of strong inhibitor of drug metabolism.
[34] The inhibition is due to the fact that angelicin decreases the activity and expression of CYP1A1 which is regulated by aryl hydrocarbon receptors (AhR).
There are three hypotheses proposed to explain the phenomenon:[34] The phototoxic properties of angelicin were deployed by its use as a natural pesticide and disinfectant.