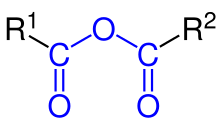
Organic acid anhydride
The low cost, however, of acetic anhydride makes it a common choice for acetylation reactions.
[citation needed] The reactivity of anhydrides can be increased by using a catalytic amount of N,N-dimethylaminopyridine ("DMAP") or even pyridine.
First, DMAP (2) attacks the anhydride (1) to form a tetrahedral intermediate, which collapses to eliminate a carboxylate ion to give amide 3.
This intermediate amide is more activated towards nucleophilic attack than the original anhydride, because dimethylaminopyridine is a better leaving group than a carboxylate.
In the final set of steps, a nucleophile (Nuc) attacks 3 to give another tetrahedral intermediate.
[7] Dianhydrides, molecules containing two acid anhydride functions, are used to synthesize polyimides and sometimes polyesters[14] and polyamides.
[citation needed] Natural products containing acid anhydrides have been isolated from animals, bacteria and fungi.
The maleidride family of fungal secondary metabolites, which possess a wide range of antibiotic and antifungal activity, are alicyclic compounds with maleic anhydride functional groups.
In some cases, the anhydride may then react with nucleophiles of other cellular components, such as at the surface of the bacterium Neisseria meningitidis or on proteins localized nearby.
The replacement of all oxygen atoms with nitrogen gives imidines, these are a rare functional group which are very prone to hydrolysis.