Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings.
Since their (inaccurate) measurements showed the proportions of carbon and hydrogen of it to be the same as in naphthalene, Laurent called it paranaphtaline in his 1835 publication of the discovery,[15] which is translated to English as paranaphthalene.
[14] Two years later, however, he decided to rename the compound to its modern name derived from Ancient Greek: ἄνθραξ, romanized: anthrax, lit.
A classic laboratory method for the preparation of anthracene is by cyclodehydration of o-methyl- or o-methylene-substituted diarylketones in the so-called Elbs reaction, for example from o-tolyl phenyl ketone.
[21] In any solvent except water,[22] anthracene photodimerizes by the action of UV light: The dimer, called dianthracene (or sometimes paranthracene), is connected by a pair of new carbon-carbon bonds, the result of the [4+4] cycloaddition.
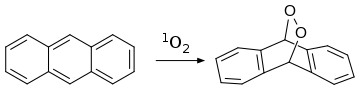
[23][24] Anthracene also reacts with dienophile singlet oxygen in a [4+2]-cycloaddition (Diels–Alder reaction): Chemical oxidation occurs readily, giving anthraquinone, C14H8O2 (below), for example using hydrogen peroxide and vanadyl acetylacetonate.
[28] Plastics, such as polyvinyltoluene, can be doped with anthracene to produce an approximately water-equivalent scintillator in radiation therapy dosimetry.
[30] Derivatives having a hydroxyl group are 1-hydroxyanthracene and 2-hydroxyanthracene, homologous to phenol and naphthols, and hydroxyanthracene (also called anthrol, and anthracenol)[31][32] are pharmacologically active.
Many investigations indicate that anthracene is noncarcinogenic: "consistently negative findings in numerous in vitro and in vivo genotoxicity tests".