Apparent molar property
In thermodynamics, an apparent molar property of a solution component in a mixture or solution is a quantity defined with the purpose of isolating the contribution of each component to the non-ideality of the mixture.
It shows the change in the corresponding solution property (for example, volume) per mole of that component added, when all of that component is added to the solution.
It is described as apparent because it appears to represent the molar property of that component in solution, provided that the properties of the other solution components are assumed to remain constant during the addition.
However this assumption is often not justified, since the values of apparent molar properties of a component may be quite different from its molar properties in the pure state.
is the volume of the pure solvent before adding the solute and
its molar volume (at the same temperature and pressure as the solution),
However this assumption must often be considered unrealistic as shown in the examples below, so that
An apparent molar quantity can be similarly defined for the component identified as solvent
Some authors have reported apparent molar volumes of both (liquid) components of the same solution.
[1][2] This procedure can be extended to ternary and multicomponent mixtures.
Apparent quantities can also be expressed using mass instead of number of moles.
where the specific quantities are denoted with small letters.
Apparent (molar) properties are not constants (even at a given temperature), but are functions of the composition.
A relation between the apparent molar of a component of a mixture and molar mixing ratio can be obtained by dividing the definition relation to the number of moles of one component.
Thus, in the defining expression for apparent molar volume
is attributed to the pure solvent, while the "leftover" excess volume,
In fact, some aqueous electrolytes have negative apparent molar volumes: NaOH −6.7, LiOH −6.0, and Na2CO3 −6.7 cm3/mole.
The physical reason is that nearby water molecules are strongly attracted to the ions so that they occupy less space.
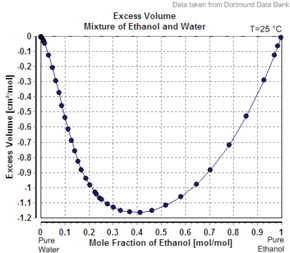
Another example of the apparent molar volume of the second component is less than its molar volume as a pure substance is the case of ethanol in water.
For example, at 20 mass percents ethanol, the solution has a volume of 1.0326 liters per kg at 20 °C, while pure water is 1.0018 L/kg (1.0018 cc/g).
However pure ethanol has a molar volume at this temperature of 58.4 cc/mole (1.27 cc/g).
If the solution were ideal, its volume would be the sum of the unmixed components.
For multicomponent solutions, apparent molar properties can be defined in several ways.
For example, partial molar volume is defined for each component i as
Another method is to treat the ternary system as pseudobinary and define the apparent molar volume of each solute with reference to a binary system containing both other components: water and the other solute.
[8] The apparent molar volumes of each of the two solutes are then The apparent molar volume of the solvent is: However, this is an unsatisfactory description of volumetric properties.
[9] The apparent molar volume of two components or solutes considered as one pseudocomponent
is not to be confused with volumes of partial binary mixtures with one common component Vij, Vjk which mixed in a certain mixing ratio form a certain ternary mixture V or Vijk.
[clarification needed] Of course the complement volume of a component in respect to other components of the mixture can be defined as a difference between the volume of the mixture and the volume of a binary submixture of a given composition like: There are situations when there is no rigorous way to define which is solvent and which is solute like in the case of liquid mixtures (say water and ethanol) that can dissolve or not a solid like sugar or salt.
In these cases apparent molar properties can and must be ascribed to all components of the mixture.