Arenavirus
A class of novel, highly divergent arenaviruses, properly known as reptarenaviruses, have also been discovered which infect snakes to produce inclusion body disease, mostly in boa constrictors.
Aseptic meningitis, a severe human disease that causes inflammation covering the brain and spinal cord, can arise from the lymphocytic choriomeningitis virus.
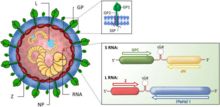
Virus particles, or virions, are pleomorphic (variable in shape) but are often spherical, with a diameter of 60–300 nm, and are covered with surface glycoprotein spikes.
This complex gene expression structure is theorized to be a primitive regulatory system, allowing the virus to control what proteins are synthesized at what point in the life cycle.
[19] The S-segment RNA is approximately 3.5 kb, and encodes the viral nucleocapsid protein (NP) and glycoprotein (GPC).
[24] The L-segment RNA is approximately 7.2 kb, and encodes the viral RNA-dependent RNA-polymerase (L) and a small RING-domain containing protein (Z).
[33] A fourth genus, Antennavirus, has also been established[34] to accommodate two arenaviruses found in striated frogfish (Antennarius striatus).
[36] Mammarenaviruses can be divided into two serogroups, which differ genetically and by geographical distribution:[37] When the virus is classified "Old World" this means it was found in the Eastern Hemisphere in places such as Europe, Asia, and Africa.
When it is found in the Western Hemisphere, in places such as Argentina, Bolivia, Venezuela, Brazil, and the United States, it is classified "New World".
Lymphocytic choriomeningitis (LCM) virus is the only mammarenavirus found worldwide because of its ubiquitous Old World host, the house mouse.
Humans' risk of contracting the Arenavirus infection is related to age, race, or sex within the degree of contact with the dried rodent excreta.
This fever is a severe illness with hemorrhagic and neurological manifestations and a case fatality of fifteen to thirty percent.
A new species of arenavirus named the Lujo virus has been linked to five patients who exhibited symptoms of viral hemorrhagic fever in South Africa.
[40] The disease originated near Lusaka, Zambia and spread to Johannesburg, South Africa, after the first patient was transported to a hospital there.
The results of genetic sequencing tests conducted by epidemiologists at Columbia University in New York City, USA, and at the Special Pathogens Branch of the Centers for Disease Control in Atlanta, USA, provided evidence that the causative agent of the disease is a virus from the family Arenaviridae, which ultimately resulted in the deaths of four out of the five infected in Zambia and South Africa during the outbreak which began in September 2008.
The current lack of a licensed vaccine and limited therapeutic options for the arenavirus make it arguably among the most neglected virus groups.
[45] Effective antiviral drugs need to be produced at a low cost, taken orally, and able to withstand tropical climates due to the regions where these infections are occurring.