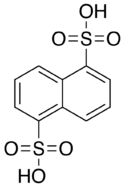
Armstrong's acid
It a colorless solid, typically obtained as the tetrahydrate.
It is named for British chemist Henry Edward Armstrong.
[2] It is prepared by disulfonation of naphthalene with oleum: Further sulfonation gives The 1,3,5-trisulfonic acid derivative.
Nitration gives nitrodisulfonic acids, which are precursors to amino derivatives.
The disodium salt is also used as an electrolyte in certain kinds of chromatography.