Atomic radius
Depending on the definition, the term may apply to atoms in condensed matter, covalently bonding in molecules, or in ionized and excited states; and its value may be obtained through experimental measurements, or computed from theoretical models.
Rather, their positions must be described as probability distributions that taper off gradually as one moves away from the nucleus, without a sharp cutoff; these are referred to as atomic orbitals or electron clouds.
This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the density of liquids and solids, the diffusion of fluids through molecular sieves, the arrangement of atoms and ions in crystals, and the size and shape of molecules.
[6] The earliest estimates of the atomic size was made by opticians in the 1830s, particularly Cauchy,[7][8] who developed models of light dispersion assuming a lattice of connected "molecules".
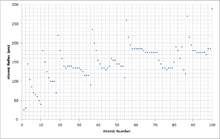
[12] Widely used definitions of atomic radius include: The following table shows empirically measured covalent radii for the elements, as published by J. C. Slater in 1964.
A similar phenomenon exists for actinides; however, the general instability of transuranic elements makes measurements for the remainder of the 5f-block difficult and for transactinides nearly impossible.
[18] This is a consequence of electrons near the strongly charged nucleus traveling at a sufficient fraction of the speed of light to gain a nontrivial amount of mass.
The following table summarizes the main phenomena that influence the atomic radius of an element: The electrons in the 4f-subshell, which is progressively filled from lanthanum (Z = 57) to ytterbium (Z = 70), are not particularly effective at shielding the increasing nuclear charge from the sub-shells further out.
In this case, it is the poor shielding capacity of the 3d-electrons which affects the atomic radii and chemistries of the elements immediately following the first row of the transition metals, from gallium (Z = 31) to bromine (Z = 35).