Tantalum
The chemical inertness and very high melting point of tantalum make it valuable for laboratory and industrial equipment such as reaction vessels and vacuum furnaces.
This conclusion was disputed in 1846 by the German chemist Heinrich Rose, who argued that there were two additional elements in the tantalite sample, and he named them after the children of Tantalus: niobium (from Niobe), and pelopium (from Pelops).
[citation needed] The differences between tantalum and niobium were demonstrated unequivocally in 1864 by Christian Wilhelm Blomstrand,[21] and Henri Etienne Sainte-Claire Deville, as well as by Louis J. Troost, who determined the empirical formulas of some of their compounds in 1865.
In the story, he had been punished after death by being condemned to stand knee-deep in water with perfect fruit growing above his head, both of which eternally tantalized him.
)[26] Anders Ekeberg wrote "This metal I call tantalum ... partly in allusion to its incapacity, when immersed in acid, to absorb any and be saturated.
[22] Tantalum is dark (blue-gray),[28] dense, ductile, very hard, easily fabricated, and highly conductive of heat and electricity.
The metal is highly resistant to corrosion by acids: at temperatures below 150 °C tantalum is almost completely immune to attack by the normally aggressive aqua regia.
The alpha phase is stable at all temperatures up to the melting point and has body-centered cubic structure with lattice constant a = 0.33029 nm at 20 °C.
An external shell of 181Ta would be irradiated by the intensive high-energy neutron flux from a hypothetical exploding nuclear weapon.
Like niobium, tantalum is barely soluble in dilute solutions of hydrochloric, sulfuric, nitric and phosphoric acids due to the precipitation of hydrous Ta(V) oxide.
During gravitational separation of the ores from placer deposits, not only is cassiterite (SnO2) found, but a small percentage of tantalite also included.
Beginning in 2007 and through 2014, the major sources of tantalum production from mines dramatically shifted to the Democratic Republic of the Congo, Rwanda, and some other African countries.
[49] Future sources of supply of tantalum, in order of estimated size, are being explored in Saudi Arabia, Egypt, Greenland, China, Mozambique, Canada, Australia, the United States, Finland, and Brazil.
Ethical questions have been raised about responsible corporate behavior, human rights, and endangering wildlife, due to the exploitation of resources such as coltan in the armed conflict regions of the Congo Basin.
[55][56][57][58] The United States Geological Survey reports in its yearbook that this region produced a little less than 1% of the world's tantalum output in 2002–2006, peaking at 10% in 2000 and 2008.
[48] USGS data published in January 2021 indicated that close to 40% of the world's tantalum mine production came from the Democratic Republic of the Congo, with another 18% coming from neighboring Rwanda and Burundi.
Although Ta occurs as various minerals, it is conveniently represented as the pentoxide, since most oxides of tantalum(V) behave similarly under these conditions.
These equations are simplified: it is suspected that bisulfate (HSO4−) and chloride compete as ligands for the Nb(V) and Ta(V) ions, when sulfuric and hydrochloric acids are used, respectively.
[61] The tantalum and niobium fluoride complexes are then removed from the aqueous solution by liquid-liquid extraction into organic solvents, such as cyclohexanone, octanol, and methyl isobutyl ketone.
This simple procedure allows the removal of most metal-containing impurities (e.g. iron, manganese, titanium, zirconium), which remain in the aqueous phase in the form of their fluorides and other complexes.
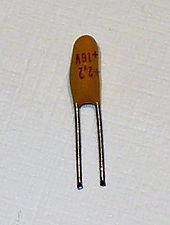
Because of the size and weight advantages, tantalum capacitors are attractive for portable telephones, personal computers, automotive electronics and cameras.
Tantalum is capable of capturing oxygen and nitrogen by forming nitrides and oxides and therefore helped to sustain the high vacuum needed for the tubes when used for internal parts such as grids and plates.
Later, Burke's team working with a team from the California Institute of Technology led by John Norton Wilson showed that tantalum, while hard enough to be fabricated into surgical tools, could also be fabricated in a form sufficiently ductile, yet still sufficiently strong to be drawn into fine threads that could be used for non-scarring sutures.
Burke's initial biological research results were confirmed and credited in greater detail by the Harvard Medical School in a series of neurological experiments using powdered tantalum implants.
[71] More than 50 years later, researchers were still refining and documenting their understanding of the basic surgical procedures developed by Burke after his pioneering discoveries.
[72] Nowadays, in spite of the cost, tantalum is still widely used in making surgical instruments and implants, and new procedures continue to be developed.
[75] The high melting point and oxidation resistance led to the use of the metal in the production of vacuum furnace parts.
Due to its high density, shaped charge and explosively formed penetrator liners have been constructed from tantalum.
Values in freshwaters fare little better, but, in all cases, they are probably below 1 ng L−1, since ‘dissolved’ concentrations in natural waters are well below most current analytical capabilities.
The metal is highly biocompatible[70] and is used for body implants and coatings, therefore attention may be focused on other elements or the physical nature of the chemical compound.