Autocatalysis
Indeed, the observation of an accelerating hydrolysis of gamma valerolactone to gamma-hydroxyvaleric acid led to the introduction of the concept of autocatalysis in 1890.
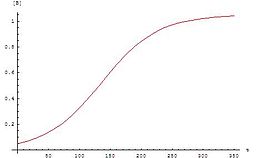
Like many radical reactions, the rate vs time plot shows a sigmoidal behavior, characteristic of autocatalysis.
The key feature of these rate equations is that they are nonlinear; the second term on the right varies as the square of the concentration of B.
These kinetic equations apply for example to the acid-catalyzed hydrolysis of some esters to carboxylic acids and alcohols.
The above equations (which do not consider the alternate pathway) for the catalyzed mechanism would imply that the concentration of acid product remains zero forever.
When seeded appropriated, saturated solutions of this salt (which is optically inactive), will produce batches of single enantiomeric crystals.
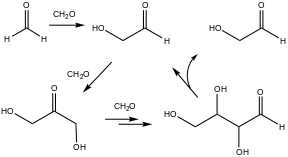
[9] An early example of autocatalysis is the formose reaction, in which formaldehyde and base produce sugars and related polyols.
Characteristic of autocatalysis, this reaction rate is extremely slow initially but accelerates with time.
This experiment demonstrated that autocatalysts could exhibit competition within a population of entities with heredity, which could be interpreted as a rudimentary form of natural selection, and that certain environmental changes (such as irradiation) could alter the chemical structure of some of these self-replicating molecules (an analog for mutation) in such ways that could either boost or interfere with its ability to react, thus boosting or interfering with its ability to replicate and spread in the population.