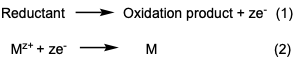Electroless deposition
[1][2][3][4] These nonconductive surfaces include plastics,[5] ceramics, and glass etc., which can then become decorative, anti-corrosive, and conductive depending on their final functions.
[8] Commonly the surface of the substrate is characterized via pXRD, SEM-EDS, and XPS which relay set parameters based their final functionality.
[6] These parameters are referred to a Key Performance Indicators crucial for a researcher’ or company's purpose.
[11] Wurtz noticed the nickel-phosphorus bath when left sitting on the benchtop spontaneously decomposed and formed a black powder.
[11][7] In 1946 the process was re-discovered by Abner Brenner and Grace E. Riddell while working at the National Bureau of Standards.
[7][12][13] They presented their discovery at the 1946 Convention of the American Electroplaters' Society (AES); a year later, at the same conference they proposed the term "electroless" for the process and described optimized bath formulations,[14] that resulted in a patent.
[17] The first commercial deposition of Ni-P was Leonhardt Plating Company in Cincinnati followed by the Kannigen Co. Ltd in Japan which revolutionized the industry.
Other metallization of substrates also include physical vapor deposition (PVD), chemical vapor deposition (CVD), and electroplating which produce thin metal films but require high temperature, vacuum, and a power source respectively.
[2][6] The plating method for Ni-P, Ni-Au, Ni-B, and Cu baths are distinct; however, the processes involve the same approach.
[2][6][11] Side product formation negatively affect the bath by poisoning the catalytic site, and disrupt the morphology of the metal nanoparticle.
[6]The electroless deposition and electroplating bath actively performs cathodic and anodic reactions at the surface of the substrate.
[2][3] The standard electrode potential of the metal and reducing agent are important as a driving force for electron exchange.
Examples are shown in Table 1., in which Zn with a lower standard potential (-0.7618 V) act as a reducing agent to copper (0.3419 V).
The standard potential of the reducing agent and metal salt is not the only determinant of the redox reaction for electroless deposition.
[citation needed] Calculation E= Ered - Eox = (-0.25 V)-(-0.50 V) = 0.25 V (spontaneous reaction) However, the atomic hydrogen mechanism did not account for the co-deposition of Ni-P.[3][6][7][14] The hydride transfer mechanism was proposed by Hersh in 1955 which accounted for the deposition of elemental phosphorus.
[6] Lukes reasoned that the hydride ion came from the hypophosphite and thus accounts for the Ni-P codeposition through a secondary reaction.
Calculation E= Ered - Eox = (-0.25 V)-(-1.65 V) = 1.45 V (spontaneous reaction) The electrochemical mechanism was also proposed by Brenner and Riddell but was later modified by others including scientists Machu and El-Gendi.
[6] They proposed that an electrolytic reaction occurred at the surface of the substrate, and H2 [11] and P [13] are by products of the Ni2+ ion reduction [10][11].
[3] The microelectronics industry including the manufacturing of circuit boards, semi-conductive devices, batteries, and sensors.
[2][3] Typical metallization of plastics includes nickel-phosphorus, nickel gold, nickel-boron, palladium, copper, and silver.
[6][9] The interference negatively affects the function of the devices; EMI sources include radiowaves, cell phones, and TV receivers.
[29][30] Elemental Ni, Cu, and Ni/Cu coating on planes absorb noise signals in the 14 Hz to 1 GHz range.