Background radiation
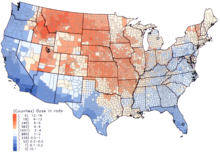
Background radiation varies with location and time, and the following table gives examples: Radioactive material is found throughout nature.
Detectable amounts occur naturally in soil, rocks, water, air, and vegetation, from which it is inhaled and ingested into the body.
[5] The International Atomic Energy Agency states: Terrestrial background radiation, for the purpose of the table above, only includes sources that remain external to the body.
The major radionuclides of concern are potassium, uranium and thorium and their decay products, some of which, like radium and radon are intensely radioactive but occur in low concentrations.
Many shorter half-life (and thus more intensely radioactive) isotopes have not decayed out of the terrestrial environment because of their on-going natural production.
Radon and its isotopes, parent radionuclides, and decay products all contribute to an average inhaled dose of 1.26 mSv/a (millisievert per year).
Radon is unevenly distributed and varies with weather, such that much higher doses apply to many areas of the world, where it represents a significant health hazard.
Concentrations over 500 times the world average have been found inside buildings in Scandinavia, the United States, Iran, and the Czech Republic.
[8] Radon is a decay product of uranium, which is relatively common in the Earth's crust, but more concentrated in ore-bearing rocks scattered around the world.
Some building materials, for example lightweight concrete with alum shale, phosphogypsum and Italian tuff, may emanate radon if they contain radium and are porous to gas.
This radiation primarily consists of positively charged ions from protons to iron and larger nuclei derived from outside the Solar System.
For example, the city of Denver in the United States (at 1650 meters elevation) receives a cosmic ray dose roughly twice that of a location at sea level.
[13] This radiation is much more intense in the upper troposphere, around 10 km altitude, and is thus of particular concern for airline crews and frequent passengers, who spend many hours per year in this environment.
During their flights airline crews typically get an additional occupational dose between 2.2 mSv (220 mrem) per year [14] and 2.19 mSv/year,[15] according to various studies.
[16] Similarly, cosmic rays cause higher background exposure in astronauts than in humans on the surface of Earth.
The production of these nuclides varies slightly with short-term variations in solar cosmic ray flux, but is considered practically constant over long scales of thousands to millions of years.
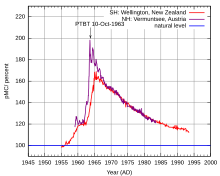
The constant production, incorporation into organisms and relatively short half-life of carbon-14 are the principles used in radiocarbon dating of ancient biological materials, such as wooden artifacts or human remains.
[7] Two of the essential elements that make up the human body, namely potassium and carbon, have radioactive isotopes that add significantly to our background radiation dose.
[21] In the world in general, exceptionally high natural background locales include Ramsar in Iran, Guarapari in Brazil, Karunagappalli in India,[22] Arkaroola in Australia[23] and Yangjiang in China.
[24] The highest level of purely natural radiation ever recorded on the Earth's surface was 90 μGy/h on a Brazilian black beach (areia preta in Portuguese) composed of monazite.
[25] This rate would convert to 0.8 Gy/a for year-round continuous exposure, but in fact the levels vary seasonally and are much lower in the nearest residences.
The highest background radiation in an inhabited area is found in Ramsar, primarily due to the use of local naturally radioactive limestone as a building material.
The 1000 most exposed residents receive an average external effective radiation dose of 6 mSv (600 mrem) per year, six times the ICRP recommended limit for exposure to the public from artificial sources.
[29] However, the recent statistical analyses discussed that there is no correlation between the risk of negative health effects and elevated level of natural background radiation.
[30] Background radiation doses in the immediate vicinity of particles of high atomic number materials, within the human body, have a small enhancement due to the photoelectric effect.
Some of this contamination is local, rendering the immediate surroundings highly radioactive, while some of it is carried longer distances as nuclear fallout; some of this material is dispersed worldwide.
The following table uses man·Sievert/GW-annum:[43] Coal plants emit radiation in the form of radioactive fly ash which is inhaled and ingested by neighbours, and incorporated into crops.
Heavy smoking results in a radiation dose of 160 mSv/year to localized spots at the bifurcations of segmental bronchi in the lungs from the decay of polonium-210.
In extreme cases it will make the instrument unusable as the background swamps the lower level of radiation from the contamination.
In such instruments the background can be continually monitored in the "Ready" state, and subtracted from any reading obtained when being used in "Measuring" mode.