Baeyer–Emmerling indole synthesis
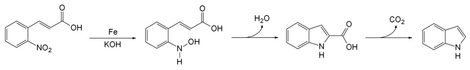
Baeyer–Emmerling indole synthesis is a method for synthesizing indole from a (substituted) ortho-nitrocinnamic acid and iron powder in strongly basic solution.
[1][2] This reaction was discovered by Adolf von Baeyer and Adolph Emmerling in 1869.
[3] [4] The reaction of iron powder with o-nitrocinnamic acid reduces the nitro group to a nitroso.
The nitrogen then condenses with a carbon on the alkene chain with loss of a molecule of water to form a ring.
Decarboxylation gives indole.