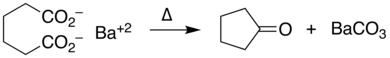Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2).
Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (EC number 4.1.1).
In the related Hammick reaction, uncatalyzed decarboxylation of a picolinic acid gives a stable carbene that attacks a carbonyl electrophile.
[13] When cannabis is heated in vacuum, the decarboxylation of tetrahydrocannabinolic acid (THCA) appears to follow first order kinetics.
At 10-degree increments from 100 to 140 °C, half of the THCA is consumed in 30, 11, 6, 3, and 2 minutes; hence the rate constant follows Arrhenius' law, ranging between 10−8 and 10−5 in a linear log-log relationship with inverse temperature.
Therefore, it was concluded that this reaction, conducted in the solid phase in plant material with a high fraction of carboxylic acids, follows a pseudo first order kinetics in which a nearby carboxylic acid precipitates without affecting the observed rate constant.
Both intermediates involve protonation of the alpha carbon, disrupting one of the double bonds of the aromatic ring and permitting the beta-keto group (which takes the form of an enol in THCA and THC) to participate in decarboxylation.