Barium titanate
Barium titanate appears white as a powder and is transparent when prepared as large crystals.
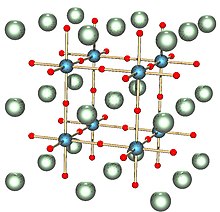
The high temperature cubic phase is easiest to describe, as it consists of regular corner-sharing octahedral TiO6 units that define a cube with O vertices and Ti-O-Ti edges.
[7] Fully dense nanocrystalline barium titanate has 40% higher permittivity than the same material prepared in classic ways.
[8] The addition of inclusions of barium titanate to tin has been shown to produce a bulk material with a higher viscoelastic stiffness than that of diamonds.
Polycrystalline barium titanate has a positive temperature coefficient of resistance, making it a useful material for thermistors and self-regulating electric heating systems.
[16] Thin films of barium titanate display electrooptic modulation to frequencies over 40 GHz.
[17] The pyroelectric and ferroelectric properties of barium titanate are used in some types of uncooled sensors for thermal cameras.
Barium titanate is widely used in thermistors and positive temperature coefficient heating elements.
[20] Due to their elevated biocompatibility, barium titanate nanoparticles (BTNPs) have been recently employed as nanocarriers for drug delivery.
[21] Magnetoelectric effect of giant strengths have been reported in thin films grown on barium titanate substrates.