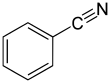
Benzonitrile
This aromatic organic compound is a colorless liquid with a sweet bitter almond odour.
[1] In the laboratory it can be prepared by the dehydration of benzamide or benzaldehyde oxime[2] or by the Rosenmund–von Braun reaction using cuprous cyanide or NaCN/DMSO and bromobenzene.
Hydrogenation of benzonitrile in principle gives benzylamine, but owing to transamination, dibenzylamine and tribenzylamine are also produced.
[4] Benzonitrile forms coordination complexes with transition metals that are both soluble in organic solvents and conveniently labile.
He deduced its structure from the already known analogue reaction of ammonium formate yielding hydrogen cyanide (formonitrile).