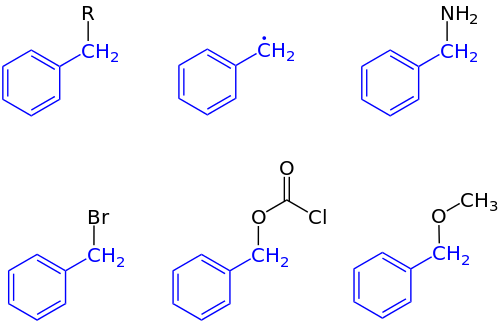
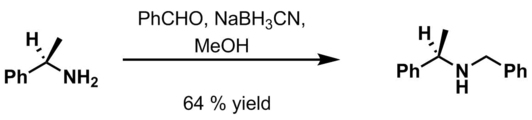Benzyl group
The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic ring.
None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of reaction mechanisms and may exist as reactive intermediates.
For related reasons, benzylic substituents exhibit enhanced reactivity, as in oxidation, free radical halogenation, or hydrogenolysis.
[6] Finally, the complex of chromium trioxide and 3,5-dimethylpyrazole (CrO3−dmpyz) will selectively oxidize a benzylic methylene group to a carbonyl: (ArCH2R → ArC(O)R).
Their installation and especially their removal require relatively harsh conditions, so benzyl is not typically preferred for protection.
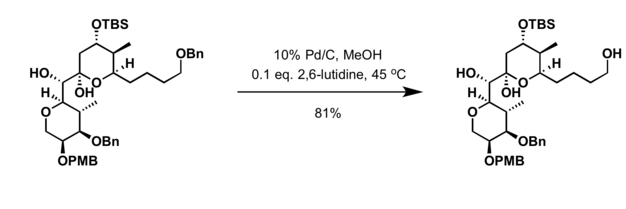
[9] Benzyl is commonly used in organic synthesis as a robust protecting group for alcohols and carboxylic acids.