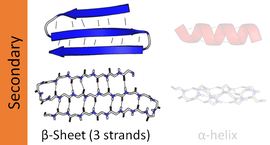
Beta sheet
A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation.
The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, Alzheimer's disease and other proteinopathies.
[2] The twist is often associated with alternating fluctuations in the dihedral angles to prevent the individual β-strands in a larger sheet from splaying apart.
The hydrogen bond arrangement in parallel beta sheet resembles that in an amide ring motif with 11 atoms.
Large aromatic residues (tyrosine, phenylalanine, tryptophan) and β-branched amino acids (threonine, valine, isoleucine) are favored to be found in β-strands in the middle of β-sheets.
Different types of residues (such as proline) are likely to be found in the edge strands in β-sheets, presumably to avoid the "edge-to-edge" association between proteins that might lead to aggregation and amyloid formation.
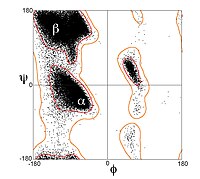
Due to the chirality of their component amino acids, all strands exhibit right-handed twist evident in most higher-order β-sheet structures.
In particular, the linking loop between two parallel strands almost always has a right-handed crossover chirality, which is strongly favored by the inherent twist of the sheet.
The vast majority of β-meander regions in proteins are found packed against other motifs or sections of the polypeptide chain, forming portions of the hydrophobic core that canonically drives formation of the folded structure.
The secondary structure of a β-sheet can be described roughly by giving the number of strands, their topology, and whether their hydrogen bonds are parallel or antiparallel.
β-pleated sheet structures are made from extended β-strand polypeptide chains, with strands linked to their neighbours by hydrogen bonds.
β-sheets in proteins may carry out low-frequency accordion-like motion as observed by the Raman spectroscopy[16] and analyzed with the quasi-continuum model.
These units "stack" atop one another in a helical fashion so that successive repetitions of the same strand hydrogen-bond with each other in a parallel orientation.
In lefthanded β-helices, the strands themselves are quite straight and untwisted; the resulting helical surfaces are nearly flat, forming a regular triangular prism shape, as shown for the 1QRE archaeal carbonic anhydrase at right.
Other examples are the lipid A synthesis enzyme LpxA and insect antifreeze proteins with a regular array of Thr sidechains on one face that mimic the structure of ice.
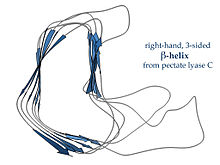
[18] Righthanded β-helices, typified by the pectate lyase enzyme shown at left or P22 phage tailspike protein, have a less regular cross-section, longer and indented on one of the sides; of the three linker loops, one is consistently just two residues long and the others are variable, often elaborated to form a binding or active site.
[19] A two-sided β-helix (right-handed) is found in some bacterial metalloproteases; its two loops are each six residues long and bind stabilizing calcium ions to maintain the integrity of the structure, using the backbone and the Asp side chain oxygens of a GGXGXD sequence motif.