Beta hairpin
[1] Beta hairpins were originally categorized solely by the number of amino acid residues in their loop sequences, such that they were named one-residue, two-residue, etc.
[2] This system, however, is somewhat ambiguous as it does not take into account whether the residues that signal the end of the hairpin are singly or doubly hydrogen bonded to one another.
This single hydrogen bond is then removed to create the tertiary hairpin; a five-residue loop with doubly bound residues.
As substitutions are the most common amino acid mutations, a protein could potentially undergo a conversion without affecting the functionality of the beta hairpin.
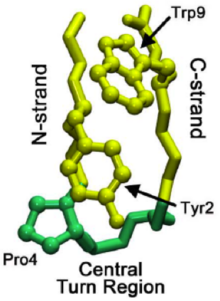
Proteins that are β-sheet rich, also called WW domains, function by adhering to proline-rich and/or phosphorylated peptides to mediate protein–protein interactions.
The "WW" refers to two tryptophan (W) residues that are conserved within the sequence and aid in the folding of the β-sheets to produce a small hydrophobic core.
[6] It is also common to find proline residues within the actual loop portion of the β-hairpin, since this amino acid is rigid and contributes to the "turn" formation.
The design of peptides that adopt β-hairpin structure (without relying on metal binding, unusual amino acids, or disulfide crosslinks) has made significant progress and yielded insights into protein dynamics.
However, this lower limit was reduced to 12 amino acids by the stability gains conferred by the incorporation of tryptophan-tryptophan cross-strand pairs.
Two nonhydrogen-bonding tryptophan pairs have been shown to interlock in a zipper-like motif, stabilizing the β-hairpin structure while still allowing it to remain water-soluble.
The NMR structure of a tryptophan zipper (trpzip) β-peptide shows the stabilizing effect of favorable interactions between adjacent indole rings.