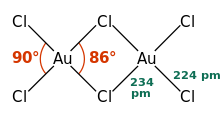
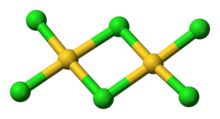
Gold(III) chloride
[12][11] Gold(III) chloride is more stable in a chlorine atmosphere and can sublime at around 200 °C without any decomposition.
For example, the reaction with potassium cyanide produces the water-soluble complex, K[Au(CN)4]:[20] Gold(III) fluoride can be also produced from gold(III) chloride by reacting it with bromine trifluoride.
[15] Gold(III) chloride reacts with benzene under mild conditions (reaction times of a few minutes at room temperature) to produce the dimeric phenylgold(III) dichloride; a variety of other arenes undergo a similar reaction:[21] Gold(III) chloride reacts with carbon monoxide in a variety of ways.
For example, the reaction of anhydrous AuCl3 and carbon monoxide under SOCl2 produces gold(I,III) chloride with Au(CO)Cl as an intermediate:[22][23] If carbon monoxide is in excess, Au(CO)Cl is produced instead.
[5] Since 2003, AuCl3 has attracted the interest of organic chemists as a mild acid catalyst for various reactions,[27] although no transformations have been commercialised.
An illustrative reaction is the hydration of terminal alkynes to produce acetyl compounds.
[28] Gold catalyses the alkylation of certain aromatic rings and the conversion of furans to phenols.
For example, a mixture of acetonitrile and gold(III) chloride catalyses the alkylation of 2-methylfuran by methyl vinyl ketone at the 5-position:[29] The efficiency of this organogold reaction is noteworthy because both the furan and the ketone are sensitive to side reactions such as polymerisation under acidic conditions.
In some cases where alkynes are present, phenols sometimes form (Ts is an abbreviation for tosyl):[29] This reaction involves a rearrangement that gives a new aromatic ring.
[33] Gold(III) chloride has been used historically in the photography industry as a sensitizer in the production of photographic films and papers.