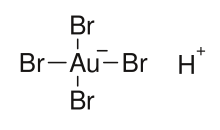Tetrabromoauric acid
Tetrabromoauric acid is an inorganic compound with the formula H[AuBr4].
It is the bromide analog of chloroauric acid.
It is generated analogously, by reacting a mixture of hydrobromic and nitric acids with elemental gold.
[1][2] The oxidation state of gold in H[AuBr4] and [AuBr4]− anion is +3.
The salts of H[AuBr4] (tetrabromoauric(III) acid) are tetrabromoaurates(III), containing [AuBr4]− anions (tetrabromoaurate(III) anions), which have square planar molecular geometry.