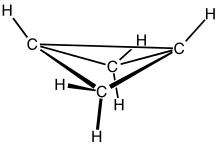
Bicyclobutane
[2] The parent hydrocarbon was prepared from 1-bromo-3-chlorocyclobutane by conversion of the bromocyclobutanecarboxylate ester,[1] followed by intramolecular Wurtz coupling using molten sodium.
[3] The intermediate 1-bromo-3-chlorocyclobutane can also be prepared via a modified Hunsdiecker reaction from 3-chlorocyclobutanecarboxylic acid using mercuric oxide and bromine:[4] A synthetic approach to bicyclobutane derivatives involves ring closure of a suitably substituted 2-bromo-1-(chloromethyl)cyclopropane with magnesium in THF.
[5] Substituted bicyclo[1.1.0]butanes can also be prepared from the reaction of iodo-bicyclo[1.1.1]pentanes with amines, thiols, and sulfinate salts.
[7] Stereochemical evidence indicates that bicyclobutane undergoes thermolysis to form 1,3-butadiene with an activation energy of 41 kcal mol−1 via a concerted pericyclic mechanism (cycloelimination, [σ2s+σ2a]).
[9] The other group reported a directed evolution approach, whereby engineered heme protein was expressed in E. coli and optimized for rate and yield of a substituted bicyclobutane derivative.
![Bicyclo[1.1.1]pentanes to Bicyclo[1.1.0]butanes](http://upload.wikimedia.org/wikipedia/commons/thumb/d/da/SchemeBCBs.svg/582px-SchemeBCBs.svg.png)