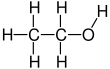
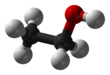
Ethanol
[16][17] It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source for lamps, stoves, and internal combustion engines.
[21] Ethanol was coined as a result of a resolution on naming alcohols and phenols that was adopted at the International Conference on Chemical Nomenclature that was held in April 1892 in Geneva, Switzerland.
[22] The term alcohol now refers to a wider class of substances in chemistry nomenclature, but in common parlance it remains the name of ethanol.
It is a medieval loan from Arabic al-kuḥl, a powdered ore of antimony used since antiquity as a cosmetic, and retained that meaning in Middle Latin.
A mutation in the ALDH2 gene that encodes for an inactive or dysfunctional form of this enzyme affects roughly 50 % of east Asian populations, contributing to the characteristic alcohol flush reaction that can cause temporary reddening of the skin as well as a number of related, and often unpleasant, symptoms of acetaldehyde toxicity.
[31] This use carries the high risk of deadly alcohol intoxication, pulmonary aspiration and vomiting, which led to use of alternatives in antiquity, such as opium and cannabis, and later diethyl ether, starting in the 1840s.
[33] Ethanol kills microorganisms by dissolving their membrane lipid bilayer and denaturing their proteins, and is effective against most bacteria, fungi and viruses.
[51] According to an industry advocacy group, ethanol as a fuel reduces harmful tailpipe emissions of carbon monoxide, particulate matter, oxides of nitrogen, and other ozone-forming pollutants.
Brazil supports this fleet of ethanol-burning automobiles with large national infrastructure that produces ethanol from domestically grown sugarcane.
[63] Ethanol was commonly used as fuel in early bipropellant rocket (liquid-propelled) vehicles, in conjunction with an oxidizer such as liquid oxygen.
The German A-4 ballistic rocket of World War II (better known by its propaganda name V-2),[64] which is credited as having begun the space age, used ethanol as the main constituent of B-Stoff.
[67][68] Alcohols fell into general disuse as more energy-dense rocket fuels were developed,[66] although ethanol was used in recent experimental lightweight rocket-powered racing aircraft.
The majority of work is being conducted at a research level although there are a number of organizations at the beginning of the commercialization of ethanol fuel cells.
It is considered a universal solvent, as its molecular structure allows for the dissolving of both polar, hydrophilic and nonpolar, hydrophobic compounds.
As ethanol also has a low boiling point, it is easy to remove from a solution that has been used to dissolve other compounds, making it a popular extracting agent for botanical oils.
Ethanol is found in paints, tinctures, markers, personal care products such as mouthwashes, perfumes and deodorants, and wet specimen preservatives.
Ethanol's hydroxyl group is able to participate in hydrogen bonding, rendering it more viscous and less volatile than less polar organic compounds of similar molecular weight, such as propane.
As the wine's ethanol content decreases, its surface tension increases and the thin film "beads up" and runs down the glass in channels rather than as a smooth sheet.
Although some animal species, such as the pentailed treeshrew, exhibit ethanol-seeking behaviors, most show no interest or avoidance of food sources containing ethanol.
[97] Minute quantity amounts (average 196 ppb) of endogenous ethanol and acetaldehyde were found in the exhaled breath of healthy volunteers.
The International Crops Research Institute for the Semi-Arid Tropics is investigating the possibility of growing sorghum as a source of fuel, food, and animal feed in arid parts of Asia and Africa.
[114] In an older process, first practiced on the industrial scale in 1930 by Union Carbide[115] but now almost entirely obsolete, ethylene was hydrated indirectly by reacting it with concentrated sulfuric acid to produce ethyl sulfate, which was hydrolyzed to yield ethanol and regenerate the sulfuric acid:[116] Ethanol in alcoholic beverages and fuel is produced by fermentation.
Certain species of yeast (e.g., Saccharomyces cerevisiae) metabolize sugar (namely polysaccharides), producing ethanol and carbon dioxide.
The chemical equations below summarize the conversion: Fermentation is the process of culturing yeast under favorable thermal conditions to produce alcohol.
Deployment of this technology could turn a number of cellulose-containing agricultural by-products, such as corncobs, straw, and sawdust, into renewable energy resources.
[116] Ethyl halides can, in principle, also be produced by treating ethanol with more specialized halogenating agents, such as thionyl chloride or phosphorus tribromide.
[140] However, this did not immediately lead to the isolation of ethanol, despite the development of more advanced distillation techniques in second- and third-century Roman Egypt.
[153] He gave the resulting solution to Henry Hennell, a British chemist, who found in 1826 that it contained "sulphovinic acid" (ethyl hydrogen sulfate).
[157] Ethanol was used as lamp fuel in the U.S. as early as 1840, but a tax levied on industrial alcohol during the Civil War made this use uneconomical.
[160] Ethanol has widespread use as a solvent of substances intended for human contact or consumption, including scents, flavorings, colorings, and medicines.