Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes.
[4][5] Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins,[6] and lipids[7] in real time in living systems without cellular toxicity.
[15] It launched the field of bioorthogonal chemistry as the first reaction with completely abiotic functional groups although it is no longer as widely used.
Phosphines are completely absent from living systems and do not reduce disulfide bonds despite mild reduction potential.
Attempts have been made to combat the problem of high background through the development of a fluorogenic phosphine reagents based on fluorescein and luciferin, but the intrinsic kinetics remain a limitation.
[16] Copper-free click chemistry is a bioorthogonal reaction first developed by Carolyn Bertozzi as an activated variant of an azide alkyne Huisgen cycloaddition, based on the work by Karl Barry Sharpless et al.
Unlike CuAAC, Cu-free click chemistry has been modified to be bioorthogonal by eliminating a cytotoxic copper catalyst, allowing reaction to proceed quickly and without live cell toxicity.
The classic copper-catalyzed azide-alkyne cycloaddition has been an extremely fast and effective click reaction for bioconjugation, but it is not suitable for use in live cells due to the toxicity of Cu(I) ions.
However, it has been found that different ligand environments of complexes can still affect metabolism and uptake, introducing an unwelcome perturbation in cellular function.
The ambivalent nature of the 1,3-dipole should make the identification of an electrophilic or nucleophilic center on the azide impossible such that the direction of the cyclic electron flow is meaningless.
More regiospecific and less bioorthogonal requirements are best served by copper-catalyzed Huisgen cycloaddition, especially given the synthetic difficulty (compared to the addition of a terminal alkyne) of synthesizing a strained cyclooctyne.
While linear alkynes are unreactive at physiological temperatures, OCT was able readily react with azides in biological conditions while showing no toxicity.
Monofluorinated (MOFO) and difluorinated (DIFO) cyclooctynes were created to increase the rate through the addition of electron-withdrawing fluorine substituents at the propargylic position.
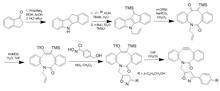
[8] DIBO (dibenzocyclooctyne) was developed as a fusion to two aryl rings, resulting in very high strain and a decrease in distortion energies.
Although calculations have predicted that mono-aryl substitution would provide an optimal balance between steric clash (with azide molecule) and strain,[20] monoarylated products have been shown to be unstable.
BARAC has sufficient rate (and sensitivity) to the extent that washing away excess probe is unnecessary to reduce background.
[21] Further adjustments variations on BARAC to produce DIBAC/ADIBO were performed to add distal ring strain and reduce sterics around the alkyne to further increase reactivity.
Keto-DIBO, in which the hydroxyl group has been converted to a ketone, has a three-fold increase in rate due to a change in ring conformation.
Although DIFO was extremely reactive in the labeling of cells, it performed poorly in mouse studies due to binding with serum albumin.
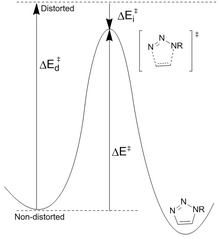
Decreasing reactant stability: Houk[23] has proposed that differences in the energy (Ed ‡) required to distort the azide and alkyne into the transition state geometries control the barrier heights for the reaction.
Calculated barriers of reaction for phenyl azide and acetylene (16.2 kcal/mol) versus cyclooctyne (8.0 kcal/mol) results in a predicted rate increase of 106.
Additional hyperconjugative interaction energy stabilization is achieved through an increase in the electronic population of the σ* due to the forming CN bond.
[24] Symmetrical cyclooctynes such as BCN (bicyclo[6.1.0]nonyne) form a single regioisomer upon cycloaddition[26] and may serve to address this problem in the future.
This allows equilibration of the alkyne prior to reaction in order to reduce artifacts as a result of concentration gradients.
Copper-free click chemistry is being explored for use in synthesizing PET imaging agents which must be made quickly with high purity and yield in order to minimize isotopic decay before the compounds can be administered.
Its primary use has been in labeling DNA and RNA in automated oligonucleotide synthesizers,[31] and polymer crosslinking in the presence of living cells.
Based on computational work by Bach, the strain energy for Z-cyclooctenes is 7.0 kcal/mol compared to 12.4 kcal/mol for cyclooctane due to a loss of two transannular interactions.
Isocyanide is a favored chemical reporter due to its small size, stability, non-toxicity, and absence in mammalian systems.
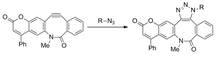
The transient nitrile imine is highly reactive for 1,3-dipolar cycloaddition due to a bent structure which reduces distortion energy.
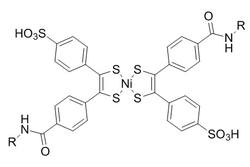
Bis(dithiobenzil)nickel(II) was chosen as a reaction partner out of a candidate screen based on reactivity.